Label: ismotic- isosorbide solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 0065-0034-08 - Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 23, 2006
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
-
DESCRIPTION
ISMOTIC® is a 45% w/v solution of isosorbide in a vanilla-mint flavored vehicle. ISMOTIC is a caramel colored aqueous solution that is chemically stable at room temperature.
Each mL contains:
Isosorbide 45% w/v (Isosorbide Concentrate 60.6%), Alcohol 0.3% w/v, Caramel, Creme de Menthe, Malic Acid, Potassium Citrate, Potassium Sorbate, Saccharin Calcium, Sodium Citrate, Sorbitol Solution, Vanilla Concentrate Imitation #20, Potassium Hydroxide (to adjust pH), and Purified Water.
DM-00
Typical analysis of electrolyte content:
4.6 meq. of Sodium/220 mL ISMOTIC Solution
0.9 meq. of Potassium/220 mL ISMOTIC Solution
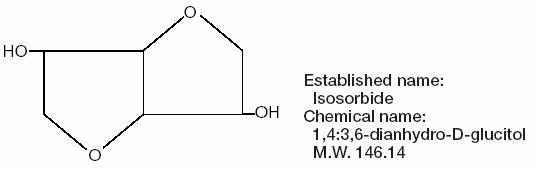
Isosorbide, the osmotic agent in ISMOTIC, is a dihydric alcohol with the formula C6H10O4 represented by the structure:
-
CLINICAL PHARMACOLOGY
Isosorbide is rapidly absorbed after oral administration. It is essentially nonmetabolized, and in the circulation, it contributes to the tonicity of the blood until it is eliminated by the kidney unchanged. While in the blood, isosorbide acts as an osmotic agent to promote redistribution of water toward the circulation with ultimate elimination in the urine. The physical action of ISMOTIC is similar to that of other osmotic drugs.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
For oral use only – not for injection. Repetitive doses should be used with caution particularly in patients with diseases associated with salt retention. Ensure that patient’s bladder has been emptied prior to surgery.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted in animals or in humans to evaluate the potential of these effects.
Pregnancy
Pregnancy Category B
Reproduction (fertility and teratology) studies have been performed in rats at doses approximately 5 times the recommended initial human dose of 1.5 gm/kg body weight and have revealed no evidence of impaired fertility or harm to the fetus due to isosorbide. Teratology studies have been performed with rabbits and rats given daily oral doses of isosorbide at 6.5 and 10 times, respectively, the recommended initial human dose during organogenesis without evidence of harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human responses, this drug should be used during pregnancy only if clearly needed.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
The recommended initial dose of isosorbide is 1.5 gm/kg body weight (equivalent to 1.5 mL /lb. of body weight). The onset of action is usually within 30 minutes while the maximum effect is expected at 1 to 1 1/2 hours. The useful dose range is 1 to 3 gm/kg body weight and the drug effect will persist up to 5 to 6 hours. Use two to four times a day as indicated. Palatability may be improved if the medication is poured over cracked ice and sipped.
RECOMMENDED DOSAGES ARE:
POUNDS MILLILITERS POUNDS MILLILITERS 100 150 155 235 105 155 160 240 110 165 165 250 115 170 170 255 120 180 175 265 125 190 180 270 130 195 185 280 135 205 190 285 140 210 195 295 145 220 200 300 150 225 -
HOW SUPPLIED
Disposable plastic bottles of 220 mL (100 gm of isosorbide/220 mL) for oral use only.
NDC 0065-0034-08
Storage: Store at 15°-30°C (59°-86°F).
CAUTION: Federal ( USA) law prohibits dispensing without prescription.
Mfd. for: Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
Mfd. by: ALCON (Puerto Rico) INC.
Humacao, Puerto Rico 00791 USA
July 1997 Printed in USA
249165-0797
-
INGREDIENTS AND APPEARANCE
ISMOTIC
isosorbide solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0065-0034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength isosorbide (UNII: WXR179L51S) (isosorbide - UNII:WXR179L51S) 450 mg in 1 mL Inactive Ingredients Ingredient Name Strength alcohol (UNII: 3K9958V90M) caramel (UNII: T9D99G2B1R) crème de menthe () malic acid (UNII: J3TZF807X5) potassium citrate (UNII: EE90ONI6FF) potassium sorbate (UNII: 1VPU26JZZ4) saccharin calcium (UNII: 5101OP7P2I) sodium citrate (UNII: 1Q73Q2JULR) sorbitol solution () vanilla concentrate imitation #20 () potassium hydroxide (UNII: WZH3C48M4T) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0034-08 220 mL in 1 BOTTLE, PLASTIC Labeler - Alcon Laboratories, Inc.
