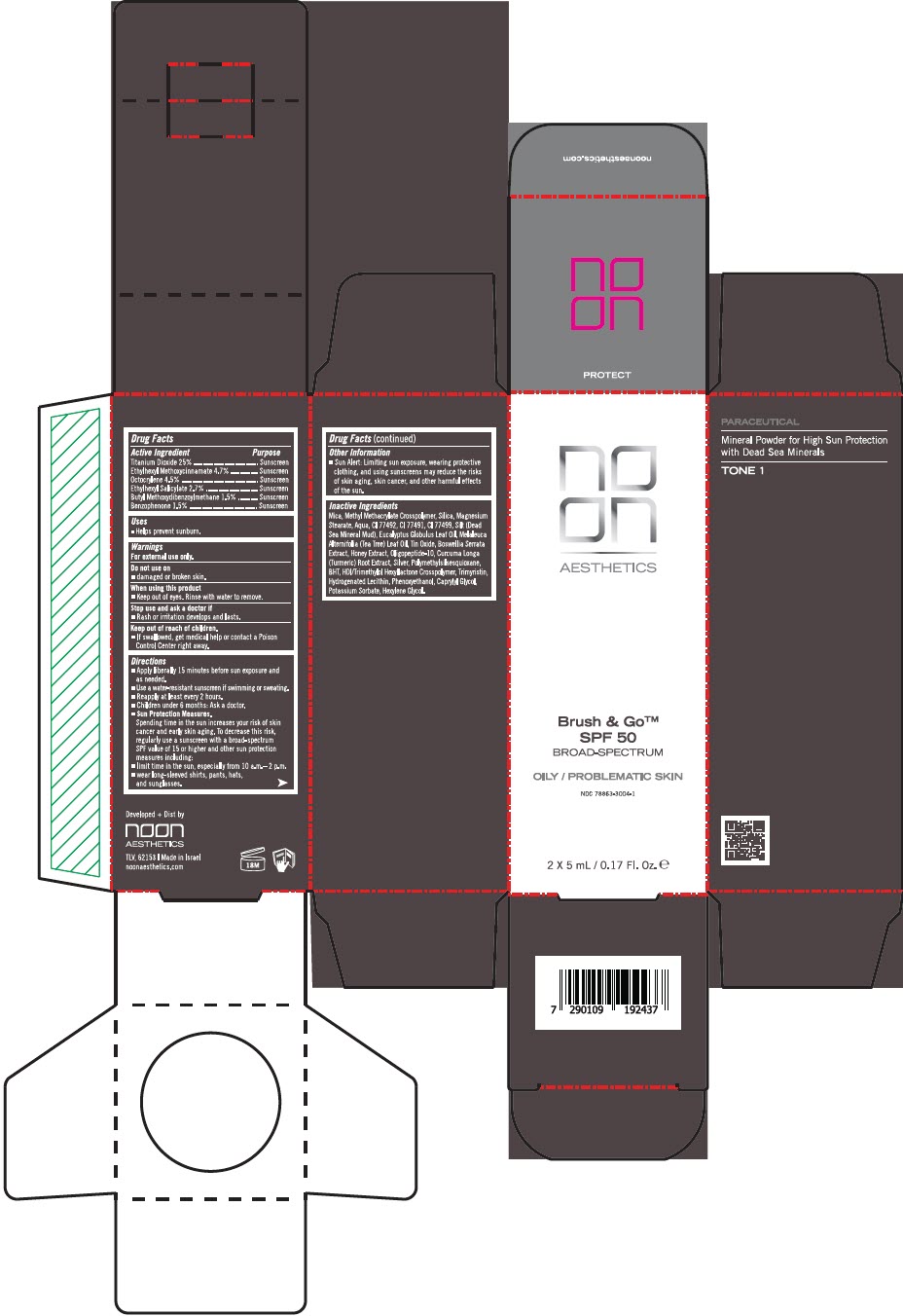
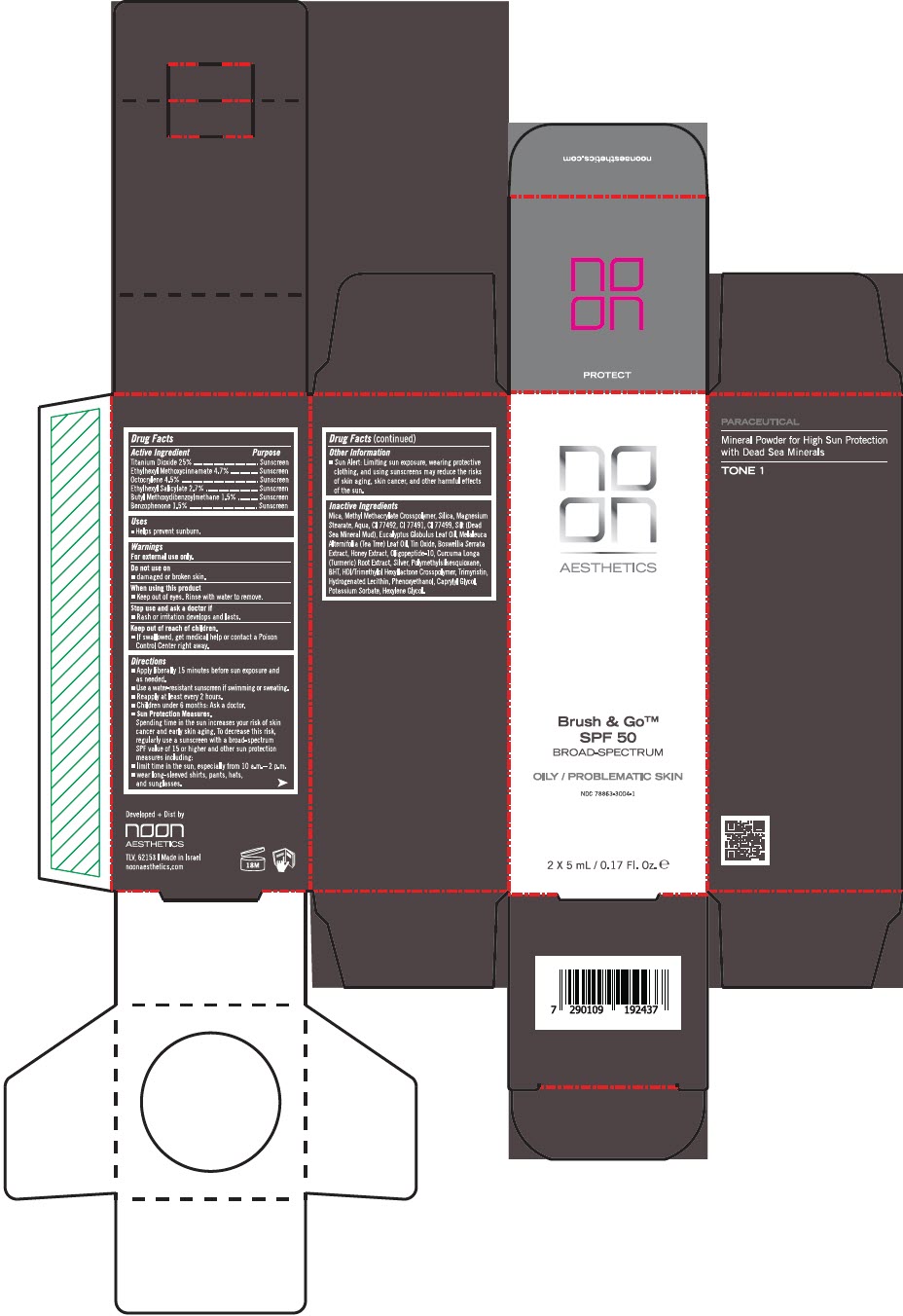
Label: BRUSH AND GO SPF 50 OILY/PROBLEMATIC SKIN BROAD SPECTRUM- titanium dioxide, octinoxate, octocrylene, octisalate, avobenzone, and oxybenzone powder
- NDC Code(s): 78863-3004-1, 78863-3004-2
- Packager: Noon Aesthetics M.R. Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure and as needed.
- Use a water-resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
- Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF value of 15 or higher and other sun protection measures including:- limit time in the sun, especially from 10 a.m.– 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses.
- Other Information
- Questions or comments?
-
Inactive Ingredients
Mica, Methyl Methacrylate Crosspolymer, Silica, Magnesium Stearate, Aqua, CI 77492, CI 77491, CI 77499, Silt (Dead Sea Mineral Mud), Eucalyptus Globulus Leaf Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Tin Oxide, Boswellia Serrata Extract, Honey Extract, Oligopeptide-10, Curcuma Longa (Turmeric) Root Extract, Silver, Polymethylsilsesquioxane, BHT, HDI/Trimethylol Hexyllactone Crosspolymer, Trimyristin, Hydrogenated Lecithin, Phenoxyethanol, Caprylyl Glycol, Potassium Sorbate, Hexylene Glycol
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 5 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
BRUSH AND GO SPF 50 OILY/PROBLEMATIC SKIN BROAD SPECTRUM
titanium dioxide, octinoxate, octocrylene, octisalate, avobenzone, and oxybenzone powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78863-3004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.75 g in 5 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.141 g in 5 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.135 g in 5 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.08 g in 5 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.045 g in 5 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.045 g in 5 mL Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) MAGNESIUM STEARATE (UNII: 70097M6I30) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EUCALYPTUS OIL (UNII: 2R04ONI662) TEA TREE OIL (UNII: VIF565UC2G) STANNOUS OXIDE (UNII: JB2MV9I3LS) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) HONEY (UNII: Y9H1V576FH) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) TURMERIC (UNII: 856YO1Z64F) SILVER (UNII: 3M4G523W1G) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) TRIMYRISTIN (UNII: 18L31PSR28) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HEXYLENE GLYCOL (UNII: KEH0A3F75J) Product Characteristics Color BROWN (LIGHT BROWN) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78863-3004-1 2 in 1 CARTON 04/01/2024 1 5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:78863-3004-2 3 in 1 CARTON 04/01/2024 2 5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 04/01/2024 Labeler - Noon Aesthetics M.R. Ltd (600185560) Establishment Name Address ID/FEI Business Operations Noon Aesthetics M.R. Ltd 600185560 MANUFACTURE(78863-3004)