Label: PURIFIED CORTROPHIN GEL- repository corticotropin injection
- NDC Code(s): 62559-860-11, 62559-860-15
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
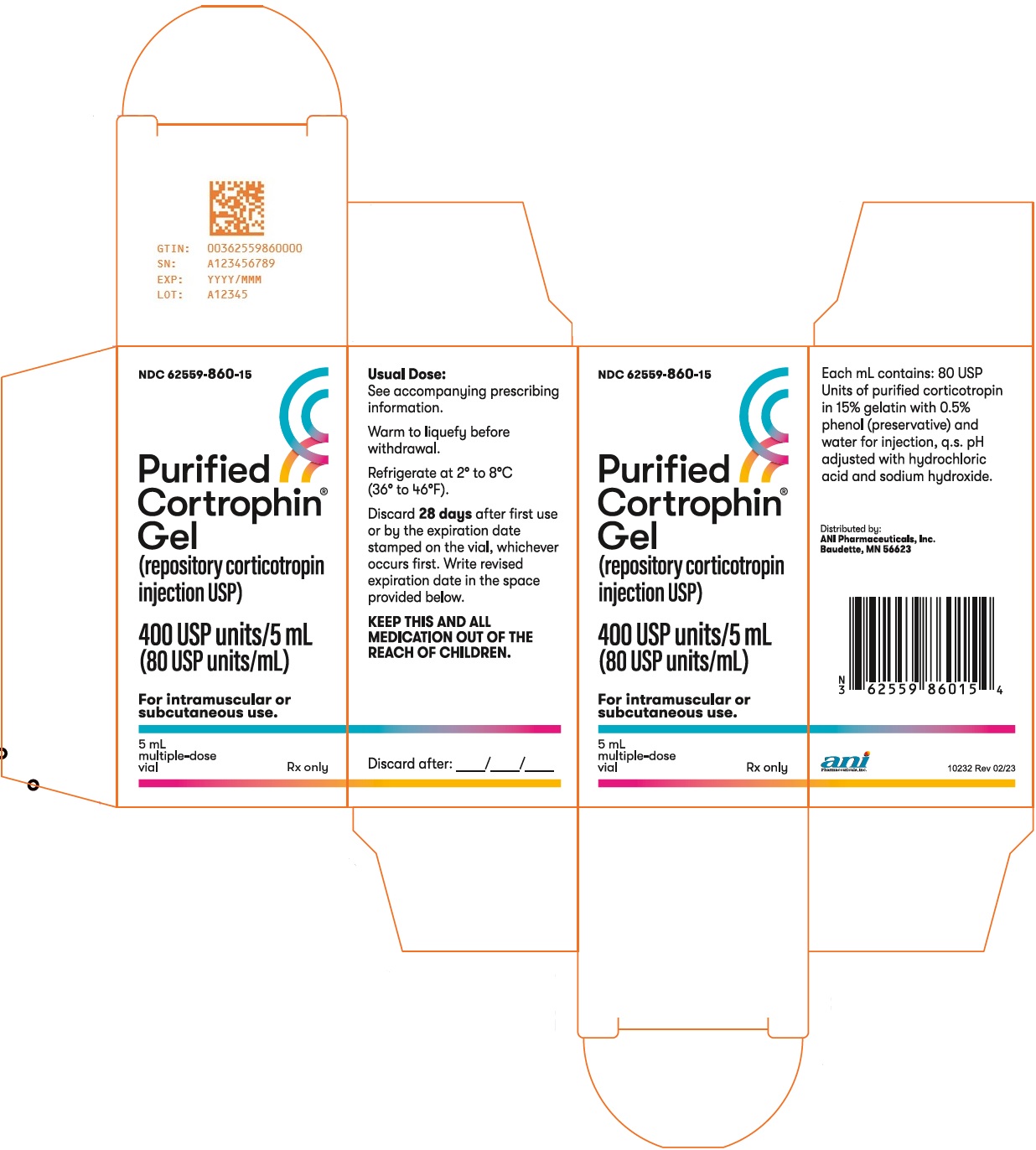
Purified Cortrophin Gel is a porcine derived purified corticotropin (ACTH) in a sterile solution of gelatin. It is made up of a complex mixture of ACTH, ACTH related peptides and other porcine pituitary derived peptides.
The drug product is a sterile preparation containing 80 USP units per mL and it contains 0.5% phenol (as preservative), 15.0% gelatin (for prolonged activity), water for injection, and the pH is adjusted with hydrochloric acid and sodium hydroxide.
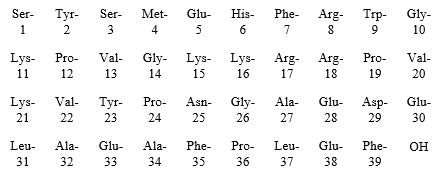
Purified Cortrophin Gel contains the porcine derived ACTH (1-39) with the following amino acid sequence:
-
CLINICAL PHARMACOLOGY
Purified Cortrophin Gel is the anterior pituitary hormone which stimulates the functioning adrenal cortex to produce and secrete adrenocortical hormones.
Following administration of a single intramuscular injection of 80 Units of Cortrophin Gel to healthy volunteers (n=20) in an open label pharmacodynamic study, the median time (range) to reach peak cortisol concentration was 8 (3-12) hours. The baseline corrected geometric mean maximum (CV%) cortisol levels were 34.52 µg/dL (28.2%).
-
INDICATIONS AND USAGE
Purified Cortrophin Gel is indicated in the following disorders:
1. Rheumatic disorders:
- As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in:
Psoriatic arthritis.
Rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy).
Ankylosing spondylitis.
Acute gouty arthritis.
2. Collagen diseases:
- During an exacerbation or as maintenance therapy in selected cases of:
Systemic lupus erythematosus.
Systemic dermatomyositis (polymyositis).
3. Dermatologic diseases:
- Severe erythema multiforme (Stevens-Johnson syndrome).
Severe psoriasis.
4. Allergic states:
- Atopic dermatitis
Serum sickness.
5. Ophthalmic diseases:
- Severe acute and chronic allergic and inflammatory processes involving the eye and its adnexa such as:
Allergic conjunctivitis.
Keratitis.
Iritis and iridocyclitis.
Diffuse posterior uveitis and choroiditis.
Optic neuritis.
Chorioretinitis.
Anterior segment inflammation.
6. Respiratory diseases:
- Symptomatic sarcoidosis.
7. Edematous states:
- To induce a diuresis or a remission of proteinuria in the nephrotic syndrome without uremia of the idiopathic type or that due to lupus erythematosus.
8. Nervous system:
- Acute exacerbations of multiple sclerosis.
-
CONTRAINDICATIONS
Purified Cortrophin Gel is contraindicated for intravenous administration.
Purified Cortrophin Gel is contraindicated in patients with scleroderma, osteoporosis, systemic fungal infections, ocular herpes simplex, recent surgery, history of or the presence of a peptic ulcer, congestive heart failure, hypertension, or sensitivity to proteins derived from porcine sources.
Purified Cortrophin Gel is contraindicated in patients with primary adrenocortical insufficiency or adrenocortical hyperfunction.
-
WARNINGS
Chronic administration of corticotropin may lead to adverse effects which are not reversible.
This product should not be administered for treatment until adrenal responsiveness has been verified with the route of administration which will be utilized during treatment, intramuscularly or subcutaneously. A rise in urinary and plasma corticosteroid values provides direct evidence of a stimulatory effect. Although the action of corticotropin is similar to that of exogenous adrenocortical steroids the quantity of adrenocorticoid may be variable. In patients who receive prolonged corticotropin therapy the additional use of rapidly acting corticosteroids before, during and after an unusual stressful situation is indicated.
Masking Symptoms of Other Diseases
Corticotropin may only suppress symptoms and signs of chronic disease without altering the natural course of the disease.
Immunogenicity Potential
Purified Cortrophin Gel is immunogenic. Limited available data suggest that a patient may develop antibodies to Purified Cortrophin Gel after chronic administration and loss of endogenous ACTH and Purified Cortrophin Gel activity. Prolonged administration of Purified Cortrophin Gel may increase the risk of hypersensitivity reactions. Sensitivity to porcine protein should be considered before starting therapy and during the course of treatment should symptoms arise.
Ophthalmic Effects
Prolonged use of corticotropin may produce posterior subcapsular cataracts and glaucoma with possible damage to the optic nerves.
Infections
Corticotropin may mask some signs of infection, and new infections including those of the eye due to fungi or viruses may appear during its use. There may be decreased resistance and inability to localize infection when corticotropin is used.
Elevated Blood Pressure, Salt and Water Retention, and Hypokalemia
Corticotropin can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. Dietary salt restriction and potassium supplementation may be necessary. Corticotropin increases calcium excretion.
Vaccination
While on corticotropin therapy, patients should not be vaccinated against smallpox. Other immunization procedures should be undertaken with caution in patients who are receiving corticotropin, especially when high doses are administered because of the possible hazards of neurological complications and lack of antibody response.
-
PRECAUTIONS
General
Patients with latent tuberculosis or tuberculin reactivity who receive corticotropin should be closely observed as reactivation of the disease may occur. During prolonged corticotropin therapy, these patients should receive chemoprophylaxis.
Skin testing should be performed prior to treatment of all patients with suspected sensitivity to porcine protein. Immediately following intramuscular or subcutaneous administration of corticotropin all patients should be observed carefully for sensitivity reactions.
Relative adrenocortical insufficiency induced by prolonged corticotropin therapy may be minimized by gradual reduction of corticotropin dosage. This type of insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress during that period, hormone therapy should be reinstituted.
There is an enhanced effect of corticotropin in patients with hypothyroidism and in those with cirrhosis.
The lowest possible dosage of corticotropin should be used to control the condition under treatment, and when reduction in dosage is possible the reduction should be gradual.
Corticotropin should be administered for treatment only when the disease is intractable to more conventional therapy. Corticotropin should be adjunctive and not the sole therapy in the treatment of a disease.
Since maximal corticotropin stimulation of the adrenals may be limited during the first few days of treatment, other drugs should be administered when an immediate therapeutic effect is desirable.
When infection is present appropriate anti-infective therapy should be administered during corticotropin and following discontinuation of corticotropin therapy.
Treatment of acute gouty arthritis should be limited to a few days. Since rebound attacks may occur when corticotropin is discontinued, conventional concomitant therapy should be administered during corticotropin treatment, and for several days after it is stopped.
Psychic derangements may appear when corticotropin is used, ranging from euphoria, insomnia, mood swings, personality changes, and depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticotropin.
Corticotropin should be used with caution in patients with diabetes, abscess, pyogenic infections, diverticulitis, renal insufficiency, and myasthenia gravis.
Growth and development of infants and children on prolonged corticotropin therapy should be carefully observed.
Although controlled clinical trials have shown ACTH to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that it affects the ultimate outcome or natural history of the disease.
Since complications of treatment with ACTH are dependent on the size of the dose and the duration of treatment, a risk/benefit decision must be made in each individual case as to dose and duration of treatment.
Drug Interactions
Aspirin should be used cautiously in conjunction with corticotropin in hypoprothrombinemia.
Pregnancy
Since fetal abnormalities have been observed in experimental animals, use of this drug in pregnancy, nursing mothers, or women of childbearing potential requires that the potential benefits of the drug be weighed against the potential hazards to the mother and embryo or fetus. Infants born of mothers who have received substantial doses of corticotropin during pregnancy should be carefully observed for signs of hypoadrenalism.
-
ADVERSE REACTIONS
Fluid and Electrolyte Disturbances
Sodium retention.
Hypokalemic alkalosis.
Fluid retention.
Calcium loss.
Potassium loss.Musculoskeletal
Muscle weakness.
Loss of muscle mass.
Steroid myopathy.
Osteoporosis.
Vertebral compression fractures.
Aseptic necrosis of femoral and humeral heads.
Pathologic fracture of long bones.Gastrointestinal
Peptic ulcer with possible perforation and hemorrhage.
Abdominal distention.
Ulcerative esophagitis.
Pancreatitis.Dermatologic
Injection site reactions.
Impaired wound healing.
Increased sweating.
Thin fragile skin.
Suppression of skin test reactions.
Petechiae and ecchymoses.
Acne.
Hyperpigmentation.
Facial erythema.Cardiovascular
Hypertension.
Congestive heart failure.
Necrotizing angiitis.Neurological
Convulsions.
Increased intracranial pressure with papilledema (pseudo-tumor cerebri), usually after treatment.
Headache.
Vertigo.Endocrine
Menstrual irregularities.
Development of Cushingoid state.
Suppression of growth in children.
Secondary adrenocortical and pituitary insufficiency, particularly in times of stress, as in trauma, surgery or illness.
Decreased carbohydrate tolerance.
Manifestations of latent diabetes mellitus.
Increased requirements for insulin or oral hypoglycemic agents in diabetics.
Hirsutism.Ophthalmic
Posterior subcapsular cataracts.
Increased intraocular pressure.
Glaucoma with possible damage to optic nerve.
Exophthalmos.Metabolic
Negative nitrogen balance due to protein catabolism.Allergic reactions
Allergic reactions manifesting as dizziness, nausea and vomiting, shock, skin reactions, especially in patients with allergic responses to proteins.Miscellaneous
Weight gain.
Abscess.
Development of antibodies and loss of stimulatory effect.To report SUSPECTED ADVERSE REACTIONS, contact ANI Pharmaceuticals, Inc. at 1-800-308-6755 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE AND ADMINISTRATION
Standard tests for verification of adrenal responsiveness to corticotropin may utilize as much as 80 units as a single injection or one or more injections of a lesser dosage. Verification tests should be performed prior to treatment with corticotropins. The test should utilize the route(s) of administration proposed for treatment. Following verification dosage should be individualized according to the disease under treatment and the general medical condition of each patient. Frequency and dose of the drug should be determined by considering severity of the disease, plasma and urine corticosteroid levels and the initial response of the patient. Only gradual change in dosage schedules should be attempted, after full drug effects have become apparent.
In the treatment of acute exacerbations of multiple sclerosis daily intramuscular doses of 80-120 units for 2-3 weeks.
The chronic administration of more than 40 units daily may be associated with uncontrollable adverse effects.
When reduction in dosage is indicated this should be accomplished gradually by either reducing the amount of each injection, or administering injections at longer intervals, or by a combination of both of the above. During reduction of dosage, careful consideration should be given to the disease being treated, the general medical condition of the patient and the duration over which corticotropin was administered.
This product may be administered subcutaneously or intramuscularly.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
- HOW SUPPLIED/ STORAGE AND HANDLING
-
Storage
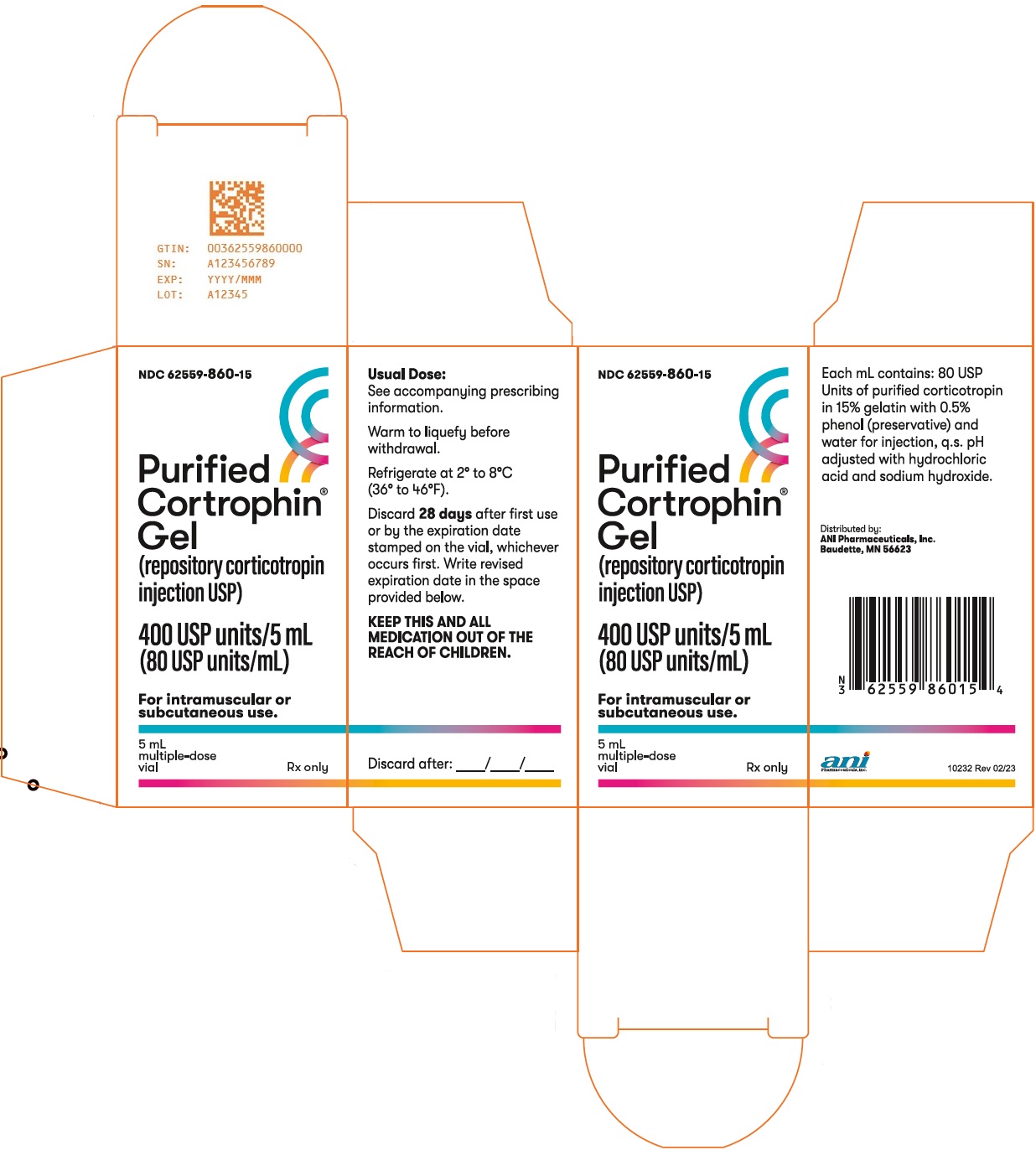
Store Purified Cortrophin Gel vials refrigerated at 2° to 8°C (36° to 46°F). After first use discard Purified Cortrophin Gel vials per the table below. Write the revised expiration date in the space provided on the carton labeling.
Package Size
Discard By
1 mL multiple-dose vial
6 months after first use or by the expiration date stamped on the vial, whichever occurs first.
5 mL multiple-dose vial
28 days after first use or by the expiration date stamped on the vial, whichever occurs first.
Distributed by:
ANI Pharmaceuticals, Inc.
Baudette, MN 56623

10233 Rev 10/23
-
Instructions for Use
Purified Cortrophin® Gel
(Repository Corticotropin Injection USP)
for intramuscular or subcutaneous useThis Instructions for Use contains information on how to inject Purified Cortrophin Gel.
Your healthcare provider should show you how to prepare and inject Purified Cortrophin Gel the right way before you inject it for the first time. Do not try to inject yourself until you have been shown the right way to give your injections by your healthcare provider.
Important information about how to inject Purified Cortrophin Gel
- •
- Purified Cortrophin Gel is given as an injection into the muscle or under the skin as directed by your healthcare provider. Do not inject it into a vein or take by mouth.
- •
- Inject Purified Cortrophin Gel exactly as your healthcare provider tells you. Your healthcare provider will tell you where to give the injection, how much to give, how often and when to give it.
- •
- Do not use Purified Cortrophin Gel until your healthcare provider has taught you how to give the injection.
Before starting, collect all of the supplies that you will need to use for preparing and injecting Purified Cortrophin Gel. You will need the following supplies:
- •
- Vial of Purified Cortrophin Gel
- •
- Syringe
- •
- Needle for withdrawal (20G or as prescribed by your healthcare provider)
- •
- Needle for injection (23G or as prescribed by your healthcare provider)
- •
- 2 alcohol pads
- •
- Cotton balls or gauze pad
- •
- Bandage (if needed)
- •
- Sharps container for throwing away used syringes and needles (see “Disposing of used needles and syringes”)
Preparing Purified Cortrophin Gel
- •
- Wash your hands well and dry with a clean towel.
- •
- Remove the vial from the refrigerator. Check the expiration date on the vial. Do not use if the expiration date has passed.
- •
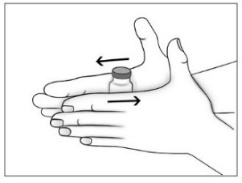
- Purified Cortrophin Gel will be a solid gel when refrigerated and it needs to be warmed to a liquid gel before injecting. Warm the solid gel in the vial by rolling between your hands for a few minutes. Do not microwave or heat on the stove.
- •
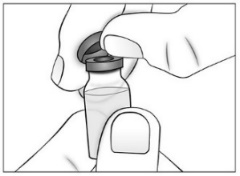
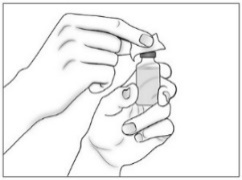
- Remove (flip off) the plastic cap from the top of the Purified Cortrophin Gel vial and throw it away in the trash. Do not put the plastic cap back on the vial.
- •
- Wipe the top of the vial rubber stopper with a new sterile alcohol pad.
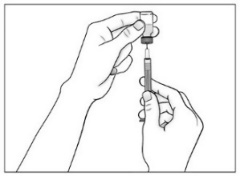
- •
- Use a new sterile 20G needle (or needle prescribed for withdrawal) and syringe to draw up the amount of Purified Cortrophin Gel your healthcare provider has told you to use.
Injecting Purified Cortrophin Gel
- •
- Prepare the skin where you are going to give the injection by wiping it with a new sterile alcohol pad. Allow to air dry.
- •
- Replace the 20G needle (or needle prescribed for withdrawal) used for drawing the Purified Cortrophin Gel from the vial with the 23G needle (or needle prescribed for injection). Do not use the 20G needle for injecting.
- •
- Give the injection the way your healthcare provider has instructed you.
- •
- There may be a little bleeding at the injection site. You can press a cotton ball or gauze pad over the injection site (do not rub).
- •
- If needed, you may cover the injection site with a bandage.
- •
- Return the vial to the refrigerator as soon as possible.
Disposing of your used needles and syringe
- •
- Put your used needles and syringe in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) your used needles and syringe in your household trash.
- •
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Storing Purified Cortrophin Gel
- •
- Store vials of Purified Cortrophin Gel in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- 1 mL vials: Throw away (discard) any 1 mL vials 6 months after first use or by the expiration date printed on the label, whichever occurs first.
- •
- 5 mL vials: Throw away (discard) any 5 mL vials 28 days after first use or by the expiration date printed on the label, whichever occurs first.
- •
- Write the revised discard by date in the space provided on the carton labeling.
Keep Purified Cortrophin Gel and all medicines out of the reach of children.
Distributed by: ANI Pharmaceuticals, Inc., Baudette, MN 56623
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: October 2023
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PURIFIED CORTROPHIN GEL
repository corticotropin injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62559-860 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CORTICOTROPIN (UNII: K0U68Q2TXA) (CORTICOTROPIN - UNII:K0U68Q2TXA) CORTICOTROPIN 80 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) PHENOL (UNII: 339NCG44TV) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62559-860-15 1 in 1 CARTON 10/29/2021 1 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 2 NDC:62559-860-11 1 in 1 CARTON 08/14/2023 2 1 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA008975 10/29/2021 Labeler - ANI Pharmaceuticals, Inc. (145588013)