Label: SALICYLIC ACID PIMPLE PATCHES- acne patches with salicylic acid patch
- NDC Code(s): 73318-1040-4
- Packager: Skin PS Brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- When using this product
- Keep out of reach of children.
- Directions
- Other Information
-
Inactive Ingredients
Acrylates Copolymer, Water, Alcohol Denat., Vinyl Caprolactam/VP/Dimethylaminoethyl, Methacrylate Copolymer, Butylene Glycol, Melaleuca Alternifolia (Tea Tree) Leaf Oil, PVP, Phenoxyethanol, Polysorbate 80, Epilobium Angustifolium Flower/Leaf/Stem Extract, Vitis Vinifera (Grape) Seed Extract, Volcanic Ash, Kaolin, Sodium Metabisulfite, Phytosphingosine, Sodium Hyaluronate.
- How to use:
- Questions or comments?
-
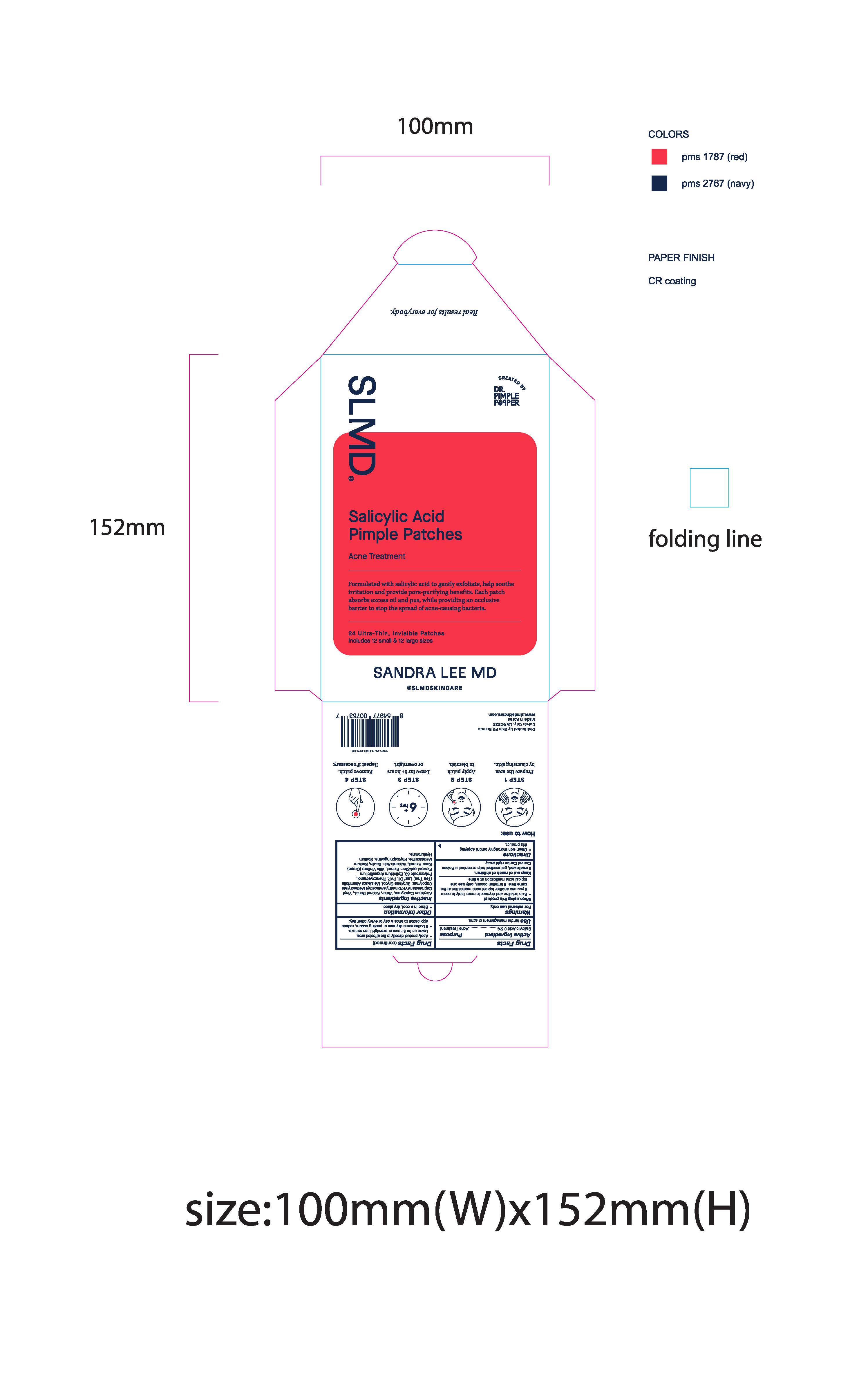
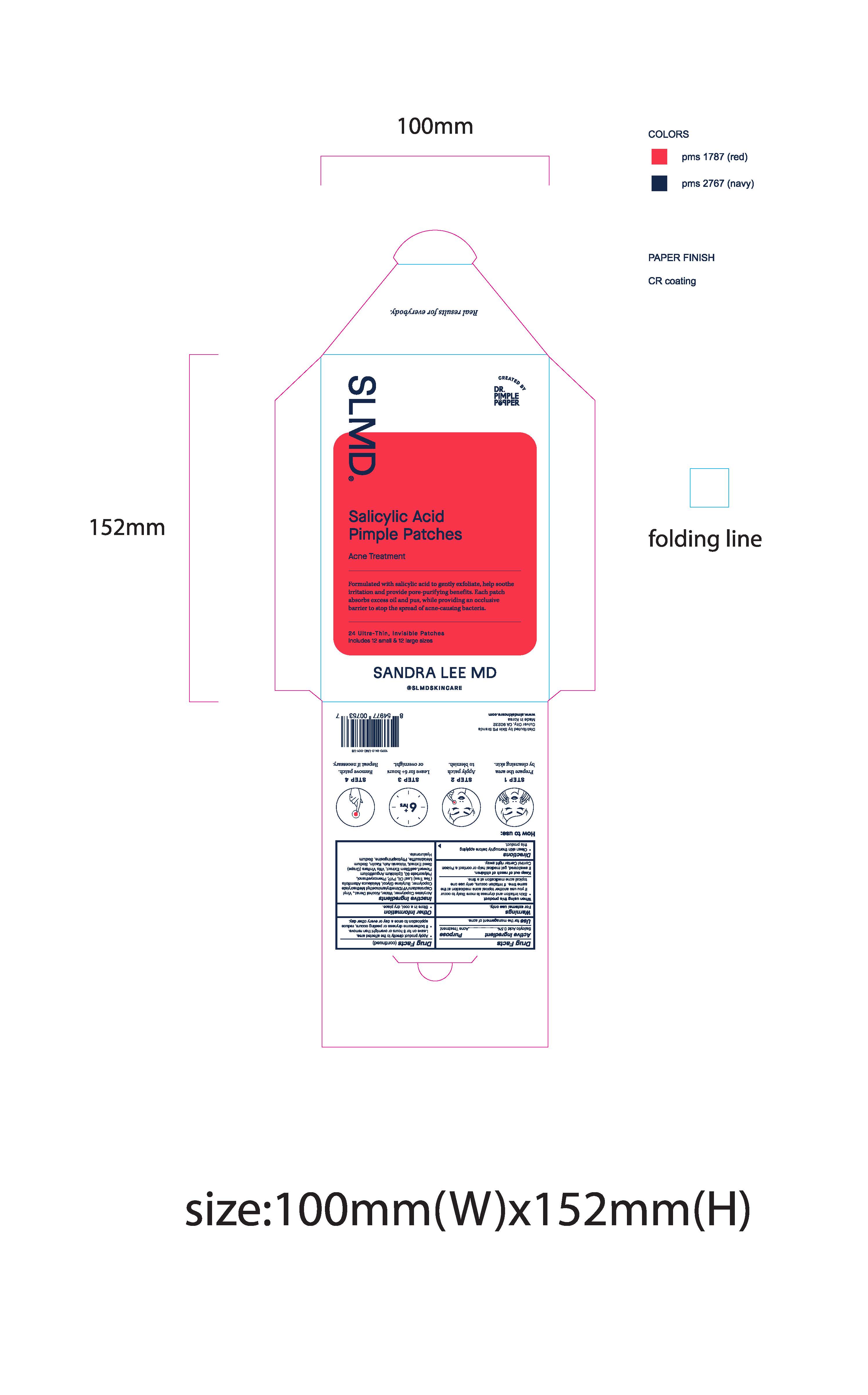
SLMD
Sandra Lee MD
Real results for everybody.
Created by Dr. Pimple Popper
SLMD
Salicylic Acid Pimple Patches
Acne Treatment
Formulated with salicylic acid to gently exfoliate, help soothe irritation and provide pore-purifying benefits. Each patch absorbs excess oil and pus, while providing an occlusive barrier to stop the spread of acne-causing bacteria.
24 Ultra-Thin, Invisible Patches
Includes 12 small & 12 large sizes
Pimple Patch Envelope:
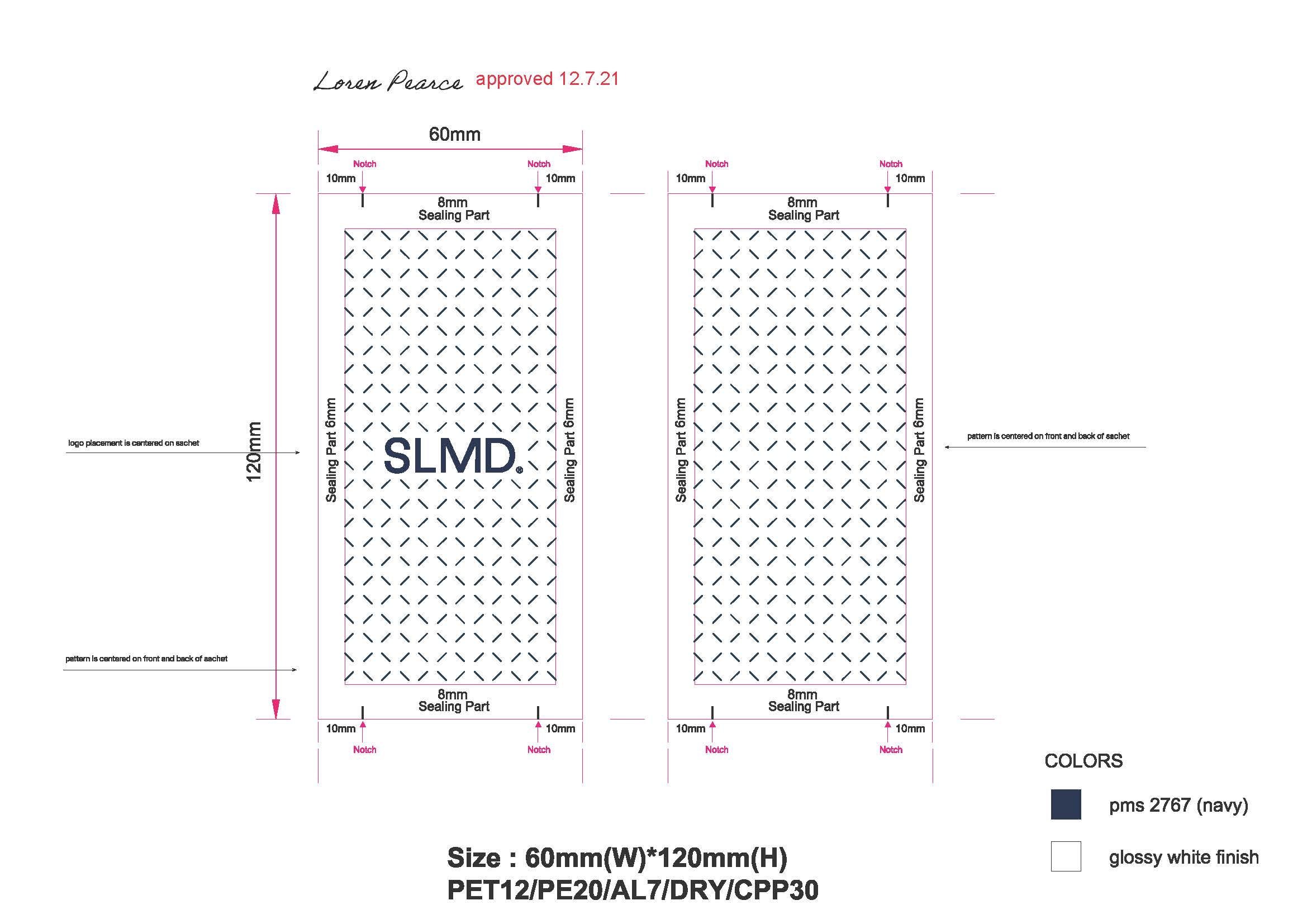
 Pimple Patch Sachet:
Pimple Patch Sachet:
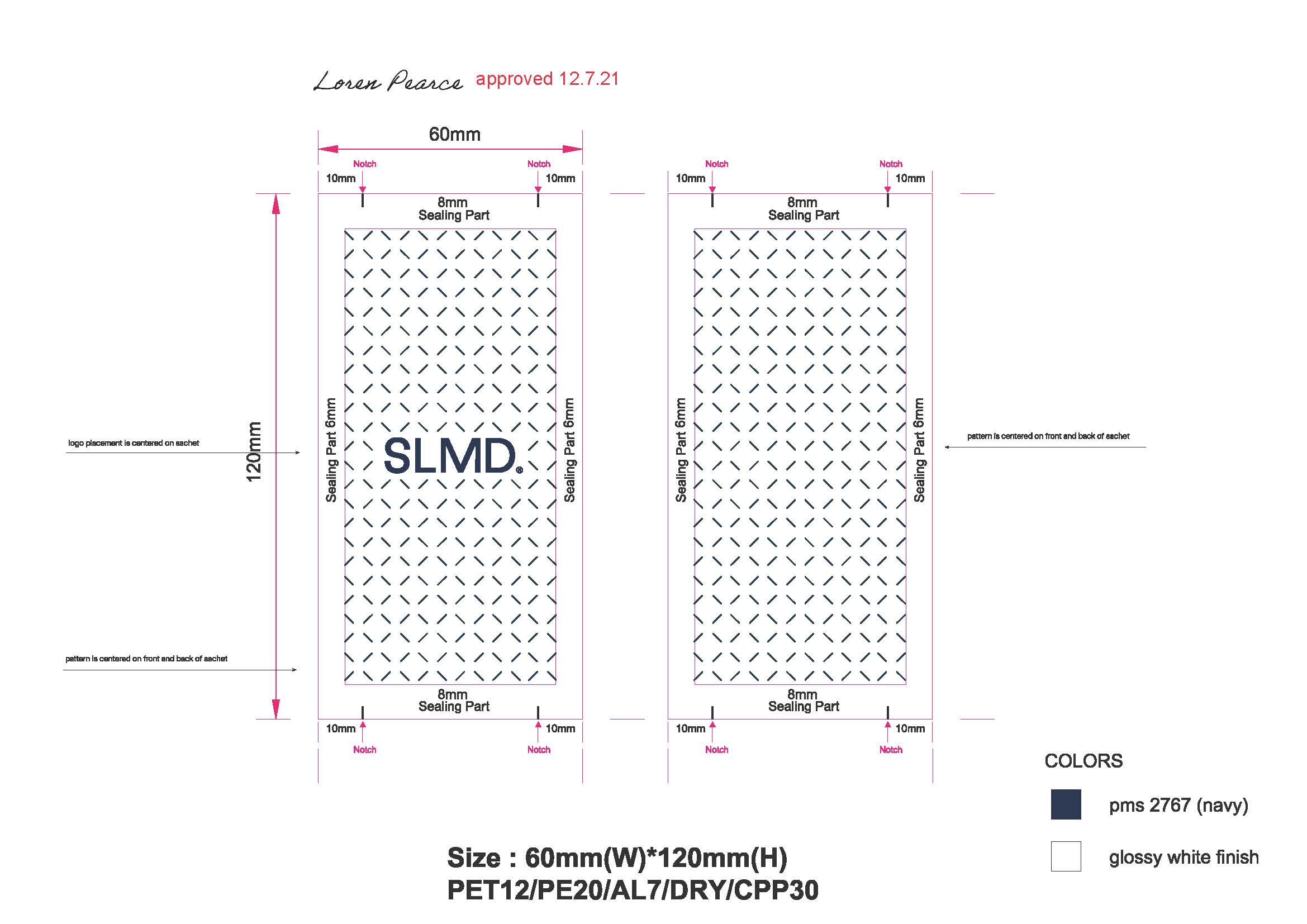
-
INGREDIENTS AND APPEARANCE
SALICYLIC ACID PIMPLE PATCHES
acne patches with salicylic acid patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73318-1040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength SODIUM METABISULFITE (UNII: 4VON5FNS3C) KAOLIN (UNII: 24H4NWX5CO) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) ACRYLIC ACID/DIMETHICONE METHACRYLATE/ETHYLHEXYL ACRYLATE COPOLYMER (UNII: E5024Y9FFQ) ALCOHOL (UNII: 3K9958V90M) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) PHENOXYETHANOL (UNII: HIE492ZZ3T) EPILOBIUM ANGUSTIFOLIUM WHOLE (UNII: C278QS9YBT) VITIS VINIFERA SEED (UNII: C34U15ICXA) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) POVIDONE K30 (UNII: U725QWY32X) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73318-1040-4 1 in 1 CARTON 01/17/2023 1 24 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 01/17/2023 Labeler - Skin PS Brands (081085221) Registrant - Skin PS Brands (081085221) Establishment Name Address ID/FEI Business Operations Taiki 688783559 manufacture(73318-1040)