Label: VIATREXX-PIGMENTATION- adrenal, azelaic, copper, cutis, cysteine, epithelial gf, fumaric acid, glutathione, glycolic acid, kalium aspariticum, lymph node, melanine, natrum oxalaceticum, natrum pyruvicum, phenylalanine, quinhydrone, succinic acid, thuja, tyrosine, vitamin c, zinc spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 63776-410-11, 63776-410-14, 63776-410-15, 63776-410-16, view more63776-410-17, 63776-410-18, 63776-410-19 - Packager: VIATREXX BIO INCORPORATED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 19, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
Active Ingredients
Adrenal (30K), Azelaic (30K), Copper (200K), Cutis (30K), Cysteine (30K), Epithelial GF (200K) Fumaric acid (30K) Glutathione (200K), Glycolic acid (30K) Kalium aspariticum (30K) Lymph node (8X, 200K), Melanine (200K), Natrum Oxalicum (30K), Natrum Pyruvicum (30K), Phenylalanine (200K), Quinhydrone (30K), Succinic acid (30K), Thuja (30K), Tyrosine (30K), Vitamin C (8X, 30K), Zinc (30K).
-
Purpose
Adrenal
Azelaic
Copper
Cutis
Cystein
Epithelial GF
Fumaric acid
Glutathione
Glycolic acid
Kalium aspariticum
Lymph node
Melanine
Natrum Oxalicum
Natrum Pyruvicum
Phenylalanine
Quinhydrone
Succinic acid
Thuja
Tyrosine
Vitamin C
ZincAdrenal support
Adaptogen
Nutritional support
Regeneration
Drainage
Regeneration
Cell respiration
Anti-oxidant
Cell respiration
Cell respiration
Immune support
Neurological support
Cell respiration
Cell respiration
Amino Acid Support
Cell respiration
Cell respiration
Drainage
Amino Acid Support
Nutritional support
Neurological support - Description
- Uses
- Warnings
- Keep this and all medicines out of reach of children.
- Dosage
- Inactive Ingredients
- Keep this and all medicines out of reach of children.
-
Product availability
Product may be acquired in 0.5, 30 ,50, 100, 250, 500, 1,000 mL bottles.
Other Information
Normal storage use.
Do not use if tamper-evident seal is broken or removed.To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Distributed by
Viatrexx Bio Incorporated
Newark, DE, USA, 19713
Manufactured by
8046255 Canada Inc
Beloeil, Qc, J3G 6S3
Date of last revision March 2019
For Questions and comments
Info@Viatrexx.com
www.Viatrexx.com -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 63776-410-11
Item: VPC0550
Viatrexx Bio Incorporated
Viatrexx-Pigmentation
0.5 mL ampule
For Oral and Topical Use
Expiry date: Lot #:
Manufactured for:
Viatrexx Bio Incorporated Newark, DE, USA, 19713
www.Viatrexx.com
Ingredients, homeopathic
See insert or www.viatrexx.com
Inactives: Alcohol 20%, Water 80%
NDC: 63776-410-14
Item: VPC0550
Viatrexx Bio Incorporated
Viatrexx-Pigmentation
30 mL spray bottle
For Oral and Topical Use
Expiry date: Lot #:
Manufactured for:
Viatrexx Bio Incorporated Newark, DE, USA, 19713
www.Viatrexx.com
Ingredients, homeopathic
See insert or www.viatrexx.com
Inactives: Alcohol 20%, Water 80%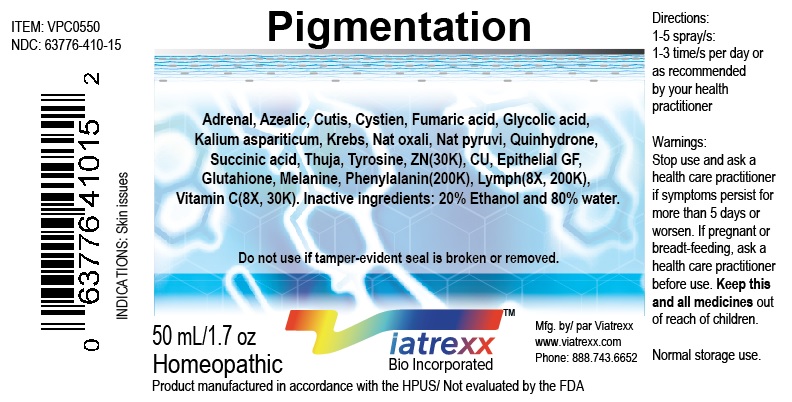 NDC: 63776-410-15
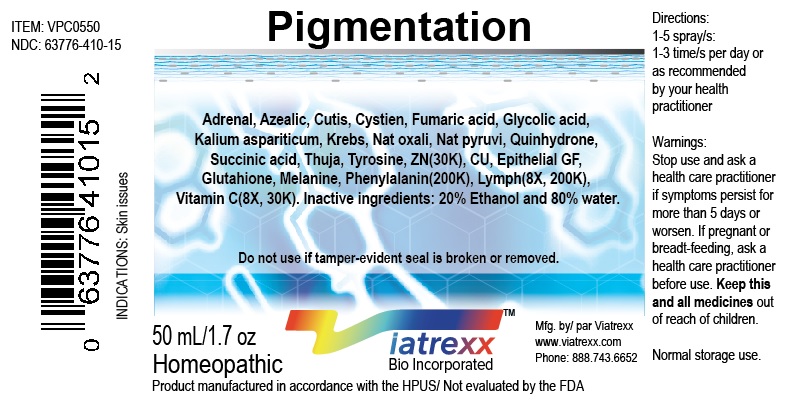
NDC: 63776-410-15
Item: VPC0550
Viatrexx Bio Incorporated
Viatrexx-Pigmentation
50 mL spray bottle
For Oral and Topical Use
Expiry date: Lot #:
Manufactured for:
Viatrexx Bio Incorporated Newark, DE, USA, 19713
www.Viatrexx.com
Ingredients, homeopathic
See insert or www.viatrexx.com
Inactives: Alcohol 20%, Water 80%NDC: 63776-410-16
Item: VPC0550
Viatrexx Bio Incorporated
Viatrexx-Pigmentation
100 mL bottle
For Oral and Topical Use
Expiry date: Lot #:
Manufactured for:
Viatrexx Bio Incorporated Newark, DE, USA, 19713
www.Viatrexx.com
Ingredients, homeopathic
See insert or www.viatrexx.com
Inactives: Alcohol 20%, Water 80%NDC: 63776-410-17
Item: VPC0550
Viatrexx Bio Incorporated
Viatrexx-Pigmentation
250 mL bottle
For Oral and Topical Use
Expiry date: Lot #:
Manufactured for:
Viatrexx Bio Incorporated Newark, DE, USA, 19713
www.Viatrexx.com
Ingredients, homeopathic
See insert or www.viatrexx.com
Inactives: Alcohol 20%, Water 80% -
INGREDIENTS AND APPEARANCE
VIATREXX-PIGMENTATION
adrenal, azelaic, copper, cutis, cysteine, epithelial gf, fumaric acid, glutathione, glycolic acid, kalium aspariticum, lymph node, melanine, natrum oxalaceticum, natrum pyruvicum, phenylalanine, quinhydrone, succinic acid, thuja, tyrosine, vitamin c, zinc sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63776-410 Route of Administration ORAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sus Scrofa Adrenal Gland (UNII: 398IYQ16YV) (Sus Scrofa Adrenal Gland - UNII:398IYQ16YV) Sus Scrofa Adrenal Gland 30 [kp_C] in 1 mL Bos Taurus Adrenal Gland (UNII: M2776SWB29) (Bos Taurus Adrenal Gland - UNII:M2776SWB29) Bos Taurus Adrenal Gland 30 [kp_C] in 1 mL Azelaic Acid (UNII: F2VW3D43YT) (Azelaic Acid - UNII:F2VW3D43YT) Azelaic Acid 30 [kp_C] in 1 mL Bos Taurus Skin (UNII: 7J12CD6O9L) (Bos Taurus Skin - UNII:7J12CD6O9L) Bos Taurus Skin 30 [kp_C] in 1 mL Copper (UNII: 789U1901C5) (Copper - UNII:789U1901C5) Copper 200 [kp_C] in 1 mL Cysteine (UNII: K848JZ4886) (Cysteine - UNII:K848JZ4886) Cysteine 30 [kp_C] in 1 mL Fumaric Acid (UNII: 88XHZ13131) (Fumaric Acid - UNII:88XHZ13131) Fumaric Acid 30 [kp_C] in 1 mL Glutathione (UNII: GAN16C9B8O) (Glutathione - UNII:GAN16C9B8O) Glutathione 200 [kp_C] in 1 mL Glycolic Acid (UNII: 0WT12SX38S) (Glycolic Acid - UNII:0WT12SX38S) Glycolic Acid 30 [kp_C] in 1 mL Potassium Aspartate (UNII: OC4598NZEQ) (Aspartic Acid - UNII:30KYC7MIAI) Potassium Aspartate 30 [kp_C] in 1 mL Nepidermin (UNII: TZK30RF92W) (Nepidermin - UNII:TZK30RF92W) Nepidermin 200 [kp_C] in 1 mL Sus Scrofa Lymph Vessel (UNII: SFL7R07748) (Sus Scrofa Lymph Vessel - UNII:SFL7R07748) Sus Scrofa Lymph Vessel 200 [kp_C] in 1 mL Bos Taurus Lymph Vessel (UNII: 85I1Z426OV) (Bos Taurus Lymph Vessel - UNII:85I1Z426OV) Bos Taurus Lymph Vessel 200 [kp_C] in 1 mL Sodium Diethyl Oxalacetate (UNII: 6CA025Y4FG) (Diethyl Oxalacetate - UNII:15S56468G7) Sodium Diethyl Oxalacetate 30 [kp_C] in 1 mL Sodium Pyruvate (UNII: POD38AIF08) (Pyruvic Acid - UNII:8558G7RUTR) Sodium Pyruvate 30 [kp_C] in 1 mL Phenylalanine (UNII: 47E5O17Y3R) (Phenylalanine - UNII:47E5O17Y3R) Phenylalanine 200 [kp_C] in 1 mL Quinhydrone (UNII: P4A66LQ3QJ) (Hydroquinone - UNII:XV74C1N1AE) Quinhydrone 30 [kp_C] in 1 mL Succinic Acid (UNII: AB6MNQ6J6L) (Succinic Acid - UNII:AB6MNQ6J6L) Succinic Acid 30 [kp_C] in 1 mL Thuja Occidentalis Leafy Twig (UNII: 1NT28V9397) (Thuja Occidentalis Leafy Twig - UNII:1NT28V9397) Thuja Occidentalis Leafy Twig 30 [kp_C] in 1 mL Tyrosine (UNII: 42HK56048U) (Tyrosine - UNII:42HK56048U) Tyrosine 30 [kp_C] in 1 mL Ascorbic Acid (UNII: PQ6CK8PD0R) (Ascorbic Acid - UNII:PQ6CK8PD0R) Ascorbic Acid 30 [kp_C] in 1 mL Zinc (UNII: J41CSQ7QDS) (Zinc - UNII:J41CSQ7QDS) Zinc 30 [kp_C] in 1 mL Melanin Synthetic (Tyrosine, Peroxide) (UNII: O0CV1RMR44) (Melanin Synthetic (Tyrosine, Peroxide) - UNII:O0CV1RMR44) Melanin Synthetic (Tyrosine, Peroxide) 200 [kp_C] in 1 mL Sus Scrofa Skin (UNII: 3EM4VW6TQN) (Sus Scrofa Skin - UNII:3EM4VW6TQN) Sus Scrofa Skin 30 [kp_C] in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63776-410-15 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/29/2019 2 NDC:63776-410-11 0.5 mL in 1 AMPULE; Type 0: Not a Combination Product 03/29/2019 3 NDC:63776-410-16 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/29/2019 4 NDC:63776-410-17 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/29/2019 5 NDC:63776-410-18 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/29/2019 6 NDC:63776-410-19 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/29/2019 7 NDC:63776-410-14 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/29/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/29/2019 Labeler - VIATREXX BIO INCORPORATED (078419880) Establishment Name Address ID/FEI Business Operations 8046255 Canada Inc 200651455 api manufacture(63776-410) , label(63776-410) , manufacture(63776-410) , pack(63776-410) , sterilize(63776-410)