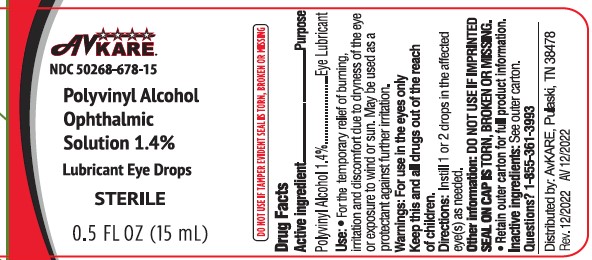
Label: POLYVINYL ALCOHOL solution/ drops
- NDC Code(s): 50268-678-15
- Packager: AvPAK
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions ?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
POLYVINYL ALCOHOL
polyvinyl alcohol solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50268-678 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYVINYL ALCOHOL (UNII: 532B59J990) (POLYVINYL ALCOHOL - UNII:532B59J990) POLYVINYL ALCOHOL 14 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50268-678-15 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/05/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 01/05/2023 Labeler - AvPAK (832926666)