Label: FLAWLESS NAILS- tolnaftate solution
- NDC Code(s): 76348-990-01, 76348-990-02
- Packager: RENU LABORATORIES, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- QUESTIONS
- STATEMENT OF IDENTITY
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
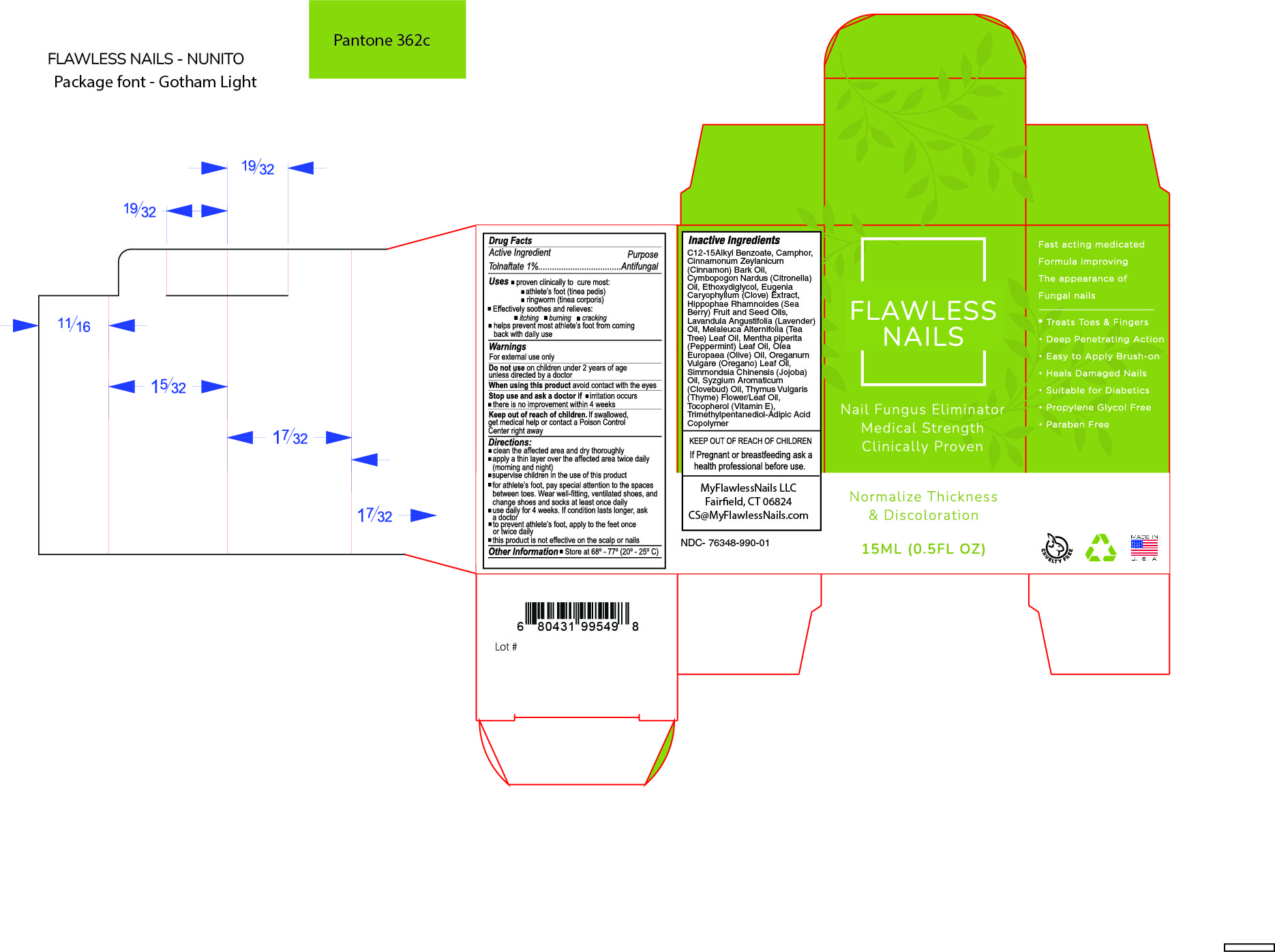
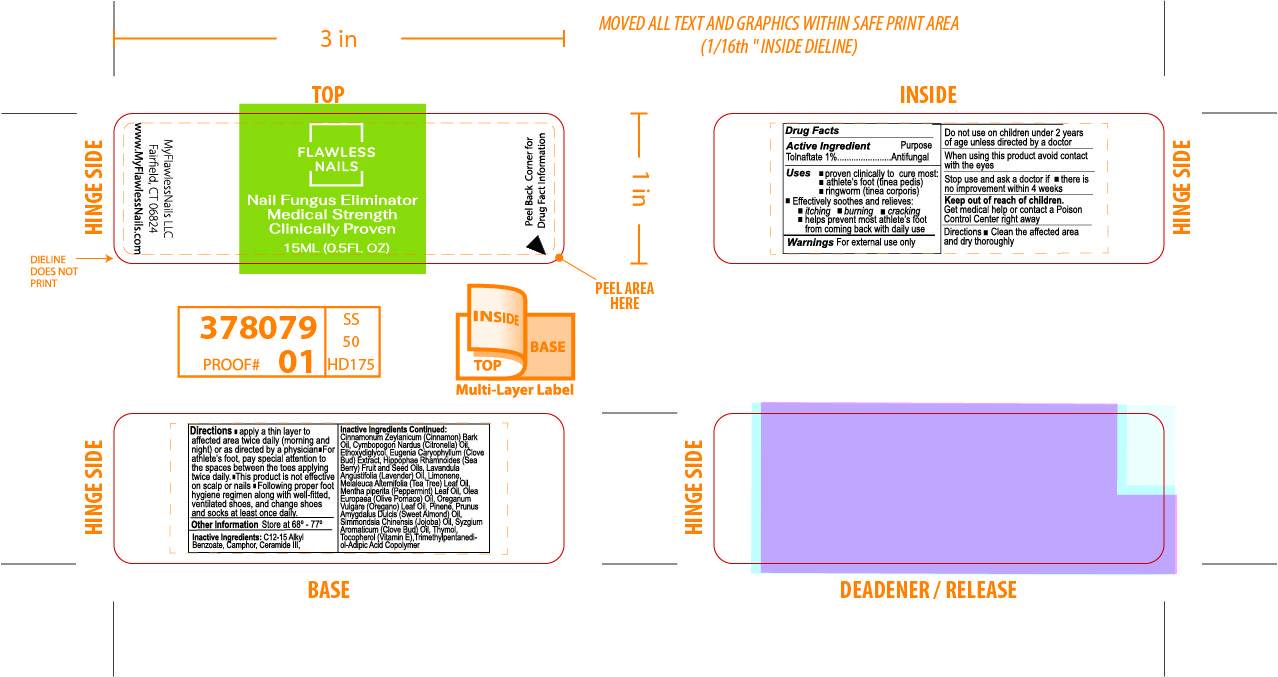
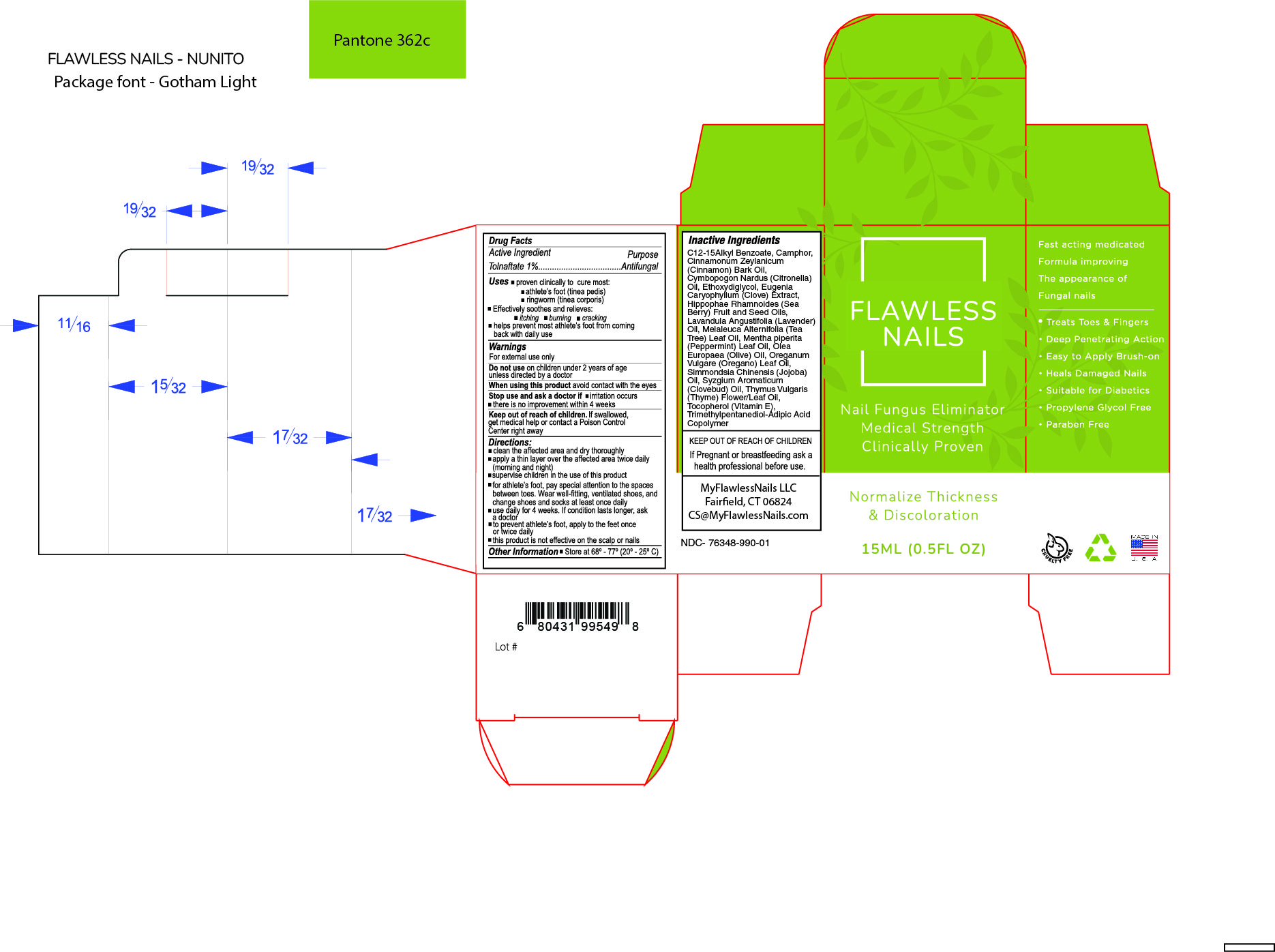
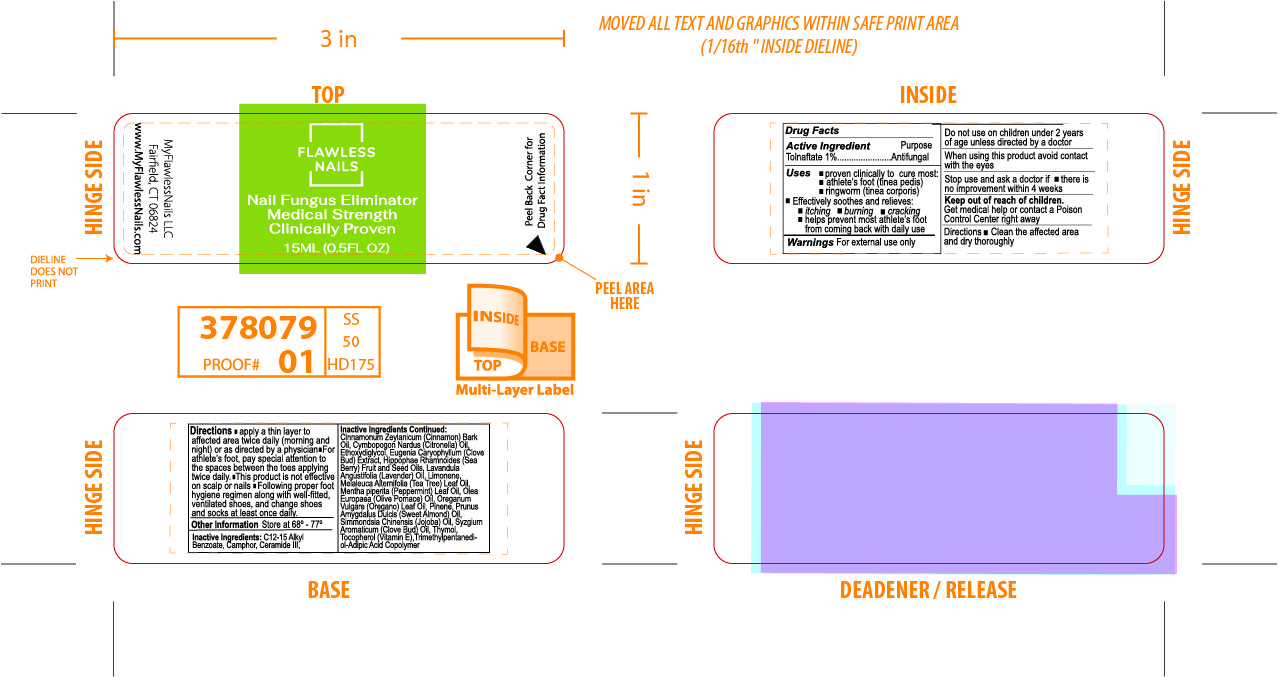
Inactive Ingredients
C12-15 Alkyl Benzoate, Camphor, Ceramide III, Cinnamonium Zeylanicum (Cinnamon Bark) Oil, Cymbopogon Nardus (Citronella) Oil, Ethoxydiglycol, Eugenia Caryophyllum (Clove Bud) Extract, Hippophae Rhamnoides (Sea Berry) Fruit and Seed Oils, Lavandula Angustifolia (Lavender) Oil, Limonene, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Mentha piperita (Peppermint) Leaf Oil,
Olea Europaea (Olive Pomace) Oil, Oreganum Vulgare (Oregano) Leaf Oil, Pinene, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Simmondsia Chinensis (Jojoba) Oil, Syzgium Aromaticum (Clove Bud) Oil, Thymol, Tocopherol Acetate, Trimethyl-Adipic Acid Copolymer.
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
Do not use on children under 2 years of age unless directed by a doctor
When using this product avoid contact with the eyes
Stop use and ask a doctor if
- there is no improvement within 4 weeks
Keep out of reach of children.
Get medical help or contact a Poison Control Center right away.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLAWLESS NAILS
tolnaftate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76348-990 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) CINNAMON BARK OIL (UNII: XE54U569EC) HIPPOPHAE RHAMNOIDES FRUIT OIL (UNII: TA4JCF9S1J) TRIMETHYLPENTANEDIOL/ADIPIC ACID/GLYCERIN CROSSPOLYMER (25000 MPA.S) (UNII: 587WKM3S9Q) PINENE (UNII: 996299PUKB) ALMOND OIL (UNII: 66YXD4DKO9) PEPPERMINT OIL (UNII: AV092KU4JH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CLOVE (UNII: K48IKT5321) LAVENDER OIL (UNII: ZBP1YXW0H8) LIMONENE, (+)- (UNII: GFD7C86Q1W) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) TEA TREE OIL (UNII: VIF565UC2G) HIPPOPHAE RHAMNOIDES SEED OIL (UNII: T53SBG6741) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) CITRONELLA OIL (UNII: QYO8Q067D0) CERAMIDE 3 (UNII: 4370DF050B) OLIVE OIL (UNII: 6UYK2W1W1E) OREGANO LEAF OIL (UNII: 7D0CGR40U1) JOJOBA OIL (UNII: 724GKU717M) CLOVE OIL (UNII: 578389D6D0) THYMOL (UNII: 3J50XA376E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76348-990-02 1 in 1 BOX 04/03/2023 1 NDC:76348-990-01 15 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M005 04/03/2023 Labeler - RENU LABORATORIES, INC. (945739449) Establishment Name Address ID/FEI Business Operations RENU LABORATORIES 945739449 manufacture(76348-990)