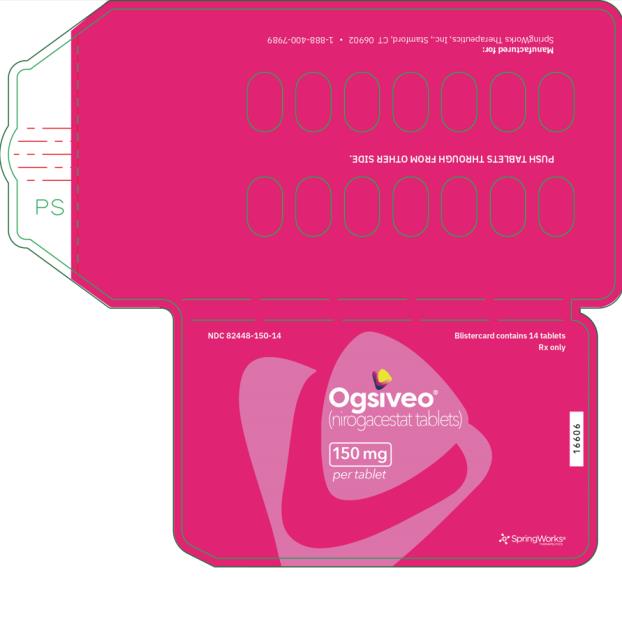
Label: OGSIVEO- nirogacestat tablet, film coated
- NDC Code(s): 82448-050-18, 82448-100-14, 82448-150-14
- Packager: SpringWorks Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated April 1, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OGSIVEO safely and effectively. See full prescribing information for OGSIVEO.
OGSIVEO® (nirogacestat) tablets, for oral use
Initial U.S. Approval: 2023
INDICATIONS AND USAGE
OGSIVEO is a gamma secretase inhibitor indicated for adult patients with progressing desmoid tumors who require systemic treatment. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 50 mg, 100 mg, and 150 mg. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Diarrhea: Severe diarrhea can occur. Monitor and dose modify for Grade 3-4 diarrhea. (5.1)
- Ovarian Toxicity: Female reproductive function and fertility may be impaired. Advise females of reproductive potential of the potential risk prior to treatment and monitor routinely. (5.2)
- Hepatotoxicity: Elevated AST and ALT can occur. Monitor AST and ALT regularly and modify dose as recommended. (5.3)
- Non-Melanoma Skin Cancers: Perform dermatologic examination prior to initiation of OGSIVEO and routinely during treatment. (5.4)
- Electrolyte Abnormalities: Monitor phosphate and potassium regularly and modify dose as recommended. (5.5)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.6, 8.1, 8.3)
ADVERSE REACTIONS
The most common (> 15 %) adverse reactions are diarrhea, ovarian toxicity, rash, nausea, fatigue, stomatitis, headache, abdominal pain, cough, alopecia, upper respiratory tract infection and dyspnea. (6.1)
The most common laboratory abnormalities (≥15%) are decreased phosphate, increased urine glucose, increased urine protein, increased AST, increased ALT, and decreased potassium. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact SpringWorks Therapeutics at 1-888-400-7989 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- Strong or moderate CYP3A inhibitors: Avoid concomitant use. (7.1)
- Strong or moderate CYP3A inducers: Avoid concomitant use. (7.1)
- Gastric acid reducing agents: Avoid concomitant use with proton pump inhibitors and H2-receptor antagonists. If concomitant use cannot be avoided, OGSIVEO administration can be staggered with antacids. (7.1)
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2023
- Diarrhea: Severe diarrhea can occur. Monitor and dose modify for Grade 3-4 diarrhea. (5.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modifications for Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Diarrhea
5.2 Ovarian Toxicity
5.3 Hepatotoxicity
5.4 Non-Melanoma Skin Cancers
5.5 Electrolyte Abnormalities
5.6 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on OGSIVEO
7.2 Effects of OGSIVEO on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Desmoid Tumor
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2
DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
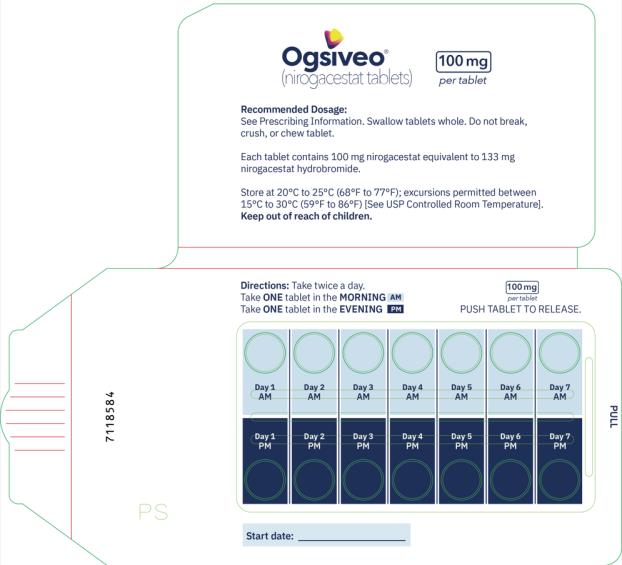
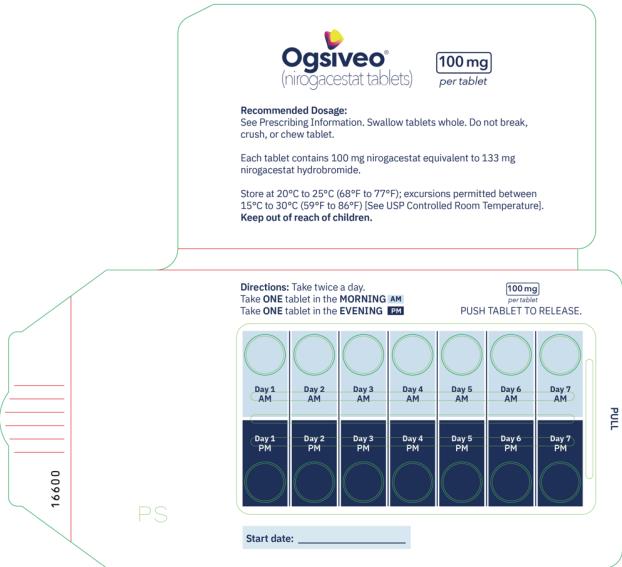
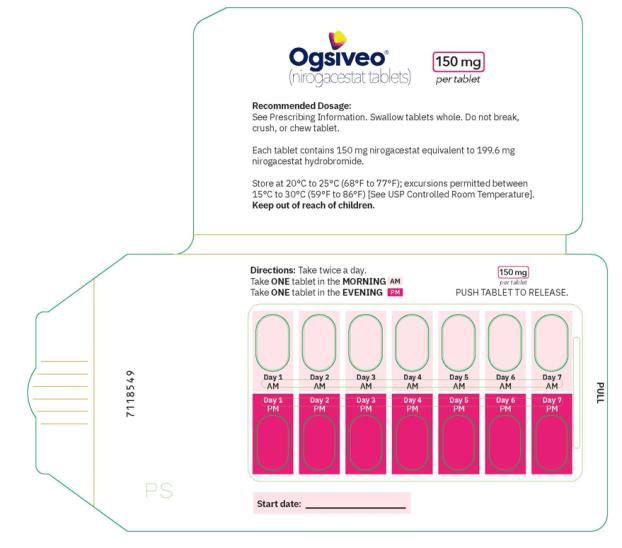
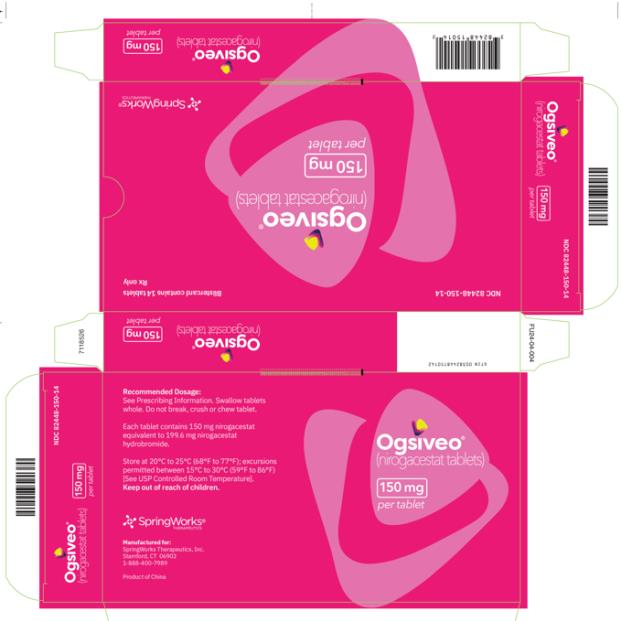
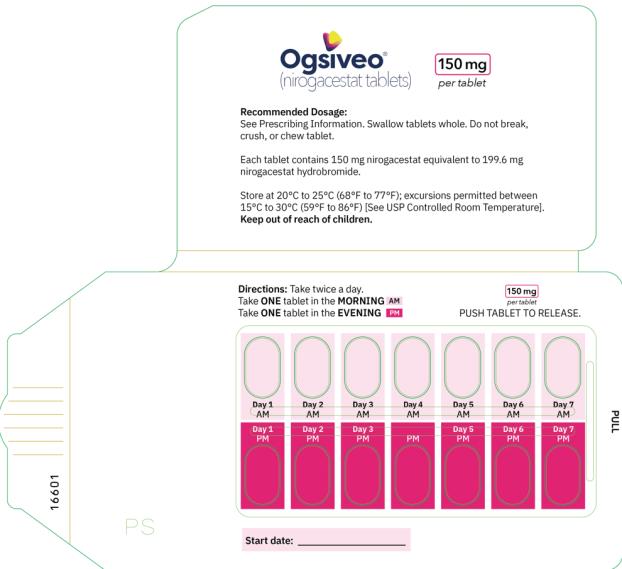
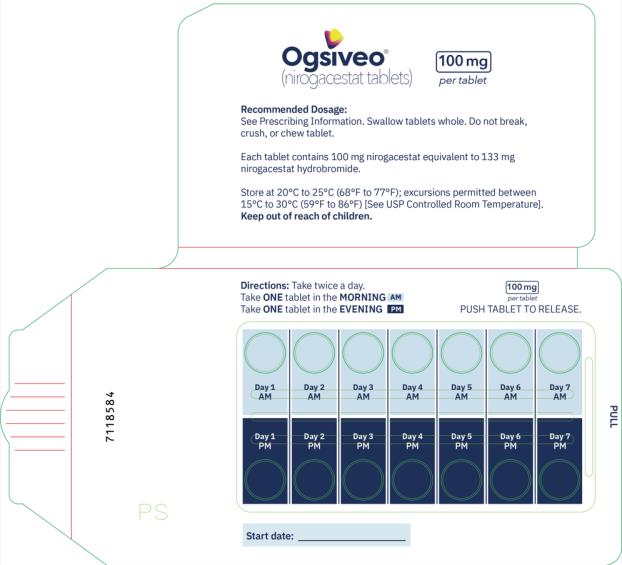
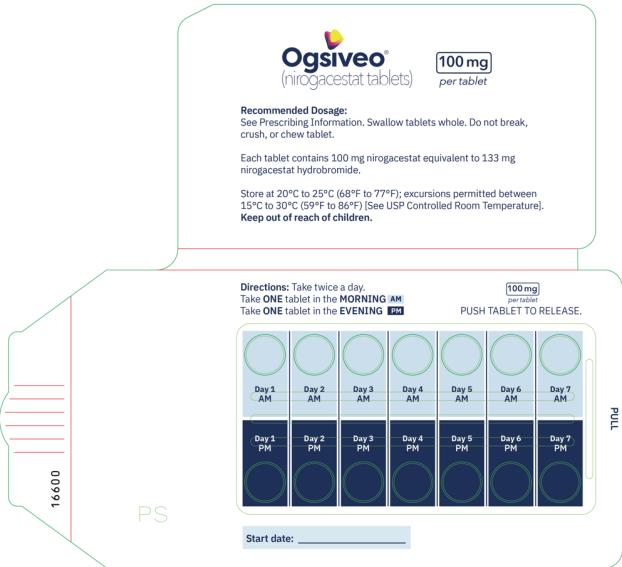
The recommended dosage of OGSIVEO is 150 mg administered orally twice daily until disease progression or unacceptable toxicity. Each 150 mg dose of OGSIVEO consists of three 50 mg tablets or one 150 mg tablet. OGSIVEO may be taken with or without food.
Instruct patients to swallow OGSIVEO tablets whole and not to break, crush, or chew prior to swallowing.
If a patient vomits or misses a dose, instruct the patient to take the next dose at its scheduled time.
2.2 Dosage Modifications for Adverse Reactions
The recommended dose modifications for OGSIVEO for selected severe adverse reactions are summarized in Table 1 [see Warnings and Precautions (5), Adverse Reactions (6)]. For other severe adverse reactions, life-threatening adverse reactions, or persistent intolerable Grade 2 adverse events, withhold drug until resolved to Grade ≤ 1 or baseline. Only restart at a dose of 100 mg twice daily after considering the potential benefit and likelihood of recurrence of the adverse reaction. Permanently discontinue OGSIVEO for recurrence of severe or life-threatening adverse reaction upon rechallenge at the reduced dose.
Table 1. Recommended Dose Modifications for Adverse Reactions Adverse Reaction Severity OGSIVEO Dosage Modifications Diarrhea persisting for ≥ 3 days despite maximal medical therapy
[see Warnings and Precautions (5.1)]Grades 3 or 4 Withhold OGSIVEO until resolved to Grade ≤ 1 or baseline, then restart at a dose of 100 mg twice daily. ALT or AST increased
[see Warnings and Precautions (5.3)]Grade 2
(≥ 3 to 5 × ULN)Withhold OGSIVEO until ALT, AST, or both are resolved to < 3 × ULN or baseline, then restart at a dose of 100 mg twice daily. Grades 3 or 4
(> 5 × ULN)Permanently discontinue. Hypophosphatemia persisting for ≥ 3 days despite maximal replacement therapy
[see Warnings and Precautions (5.5)]Grades 3 or 4 Withhold OGSIVEO until resolved to Grade ≤ 1 or baseline, then restart at a dose of 100 mg twice daily. Hypokalemia despite maximal replacement therapy
[see Warnings and Precautions (5.5)]Grades 3 or 4 Withhold OGSIVEO until resolved to Grade ≤ 1 or baseline, then restart at a dose of 100 mg twice daily. -
3
DOSAGE FORMS AND STRENGTHS
The 50 mg tablets are round, orange, film-coated, and debossed with “50” on one face. Each 50 mg tablet contains 50 mg nirogacestat.
The 100 mg tablets are round, light orange, film-coated, and debossed with “100” on one face. Each 100 mg tablet contains 100 mg nirogacestat.
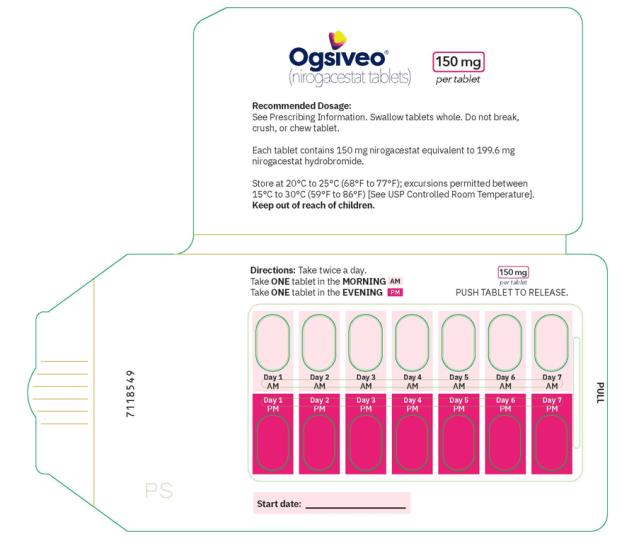
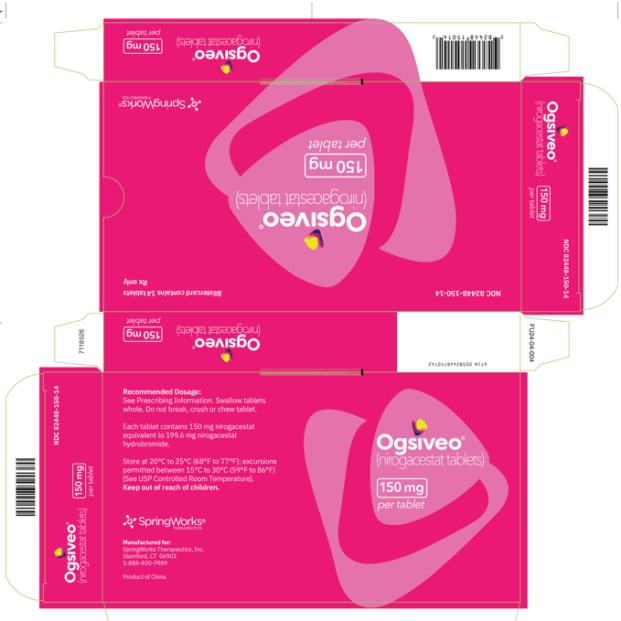
The 150 mg tablets are oval, orange yellow, film-coated, and debossed with “150” on one face. Each 150 mg tablet contains 150 mg nirogacestat
- 4 CONTRAINDICATIONS
-
5
WARNINGS AND PRECAUTIONS
5.1 Diarrhea
Diarrhea, sometimes severe, can occur in patients treated with OGSIVEO [see Adverse Reactions (6.1)].
In DeFi, diarrhea occurred in 84% of patients treated with OGSIVEO, and included Grade 3 events in 16% of patients. Median time to first diarrhea event for patients treated with OGSIVEO was 9 days (range: 2 to 434 days). Monitor patients and manage using antidiarrheal medications. Modify dose as recommended [see Dosage and Administration (2.2)].
5.2 Ovarian Toxicity
Female reproductive function and fertility may be impaired in patients being treated with OGSIVEO. Impact on fertility may depend on factors including the duration of therapy and the state of gonadal function at the time of treatment. The long-term effects of OGSIVEO on fertility have not been established. Advise patients on the potential risks for ovarian toxicity before initiating treatment with OGSIVEO [see Use in Specific Populations (8.3)]. Monitor patients for changes in menstrual cycle regularity or the development of symptoms of estrogen deficiency, including hot flashes, night sweats, and vaginal dryness.
5.3 Hepatotoxicity
ALT or AST elevations occurred in 30% and 33% of patients who received OGSIVEO in DeFi, respectively. Grade 3 ALT or AST elevations (> 5 × ULN) occurred in 6% and 2.9% of patients, respectively [see Adverse Reactions (6.1)]. Monitor liver function tests regularly and modify dose as recommended [see Dosage and Administration (2.2)].
5.4 Non-Melanoma Skin Cancers
New non-melanoma skin cancers can occur in patients treated with OGSIVEO. In DeFi, cutaneous squamous cell carcinoma and basal cell carcinoma occurred in 2.9% and 1.4% of patients, respectively [see Adverse Reactions (6.1)]. Perform dermatologic evaluations prior to initiation of OGSIVEO and routinely during treatment.
5.5 Electrolyte Abnormalities
Electrolyte abnormalities can occur in patients treated with OGSIVEO. In DeFi, these included decreased phosphate (65%) and decreased potassium (22%). Phosphate <2 mg/dL occurred in 20% of patients who received OGSIVEO. Grade 3 decreased potassium occurred in 1.4% of patients [see Adverse Reactions (6.1)]. Monitor phosphate and potassium levels regularly and supplement as necessary. Modify dose as recommended [see Dosage and Administration (2.2)].
5.6 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, OGSIVEO can cause fetal harm when administered to pregnant women. Oral administration of nirogacestat to pregnant rats during the period of organogenesis resulted in embryo-fetal toxicity and death at maternal exposures below the human exposure at the recommended dose of 150 mg twice daily. Advise pregnant women of the potential risk to a fetus. Advise females and males of reproductive potential to use effective contraception during treatment with OGSIVEO and for 1 week after the last dose [see Use in Specific Populations (8.1,8.3)].
-
6
ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Diarrhea [see Warnings and Precautions (5.1)]
- Ovarian Toxicity [see Warnings and Precautions (5.2)]
- Hepatotoxicity [see Warnings and Precautions (5.3)]
- Non-Melanoma Skin Cancers [see Warnings and Precautions (5.4)]
- Electrolyte Abnormalities [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of OGSIVEO was evaluated in 69 patients enrolled in DeFi with progressing desmoid tumor [see Clinical Studies (14)]. Patients received OGSIVEO 150 mg orally twice daily or placebo orally twice daily until disease progression or unacceptable toxicity. The median duration of exposure to OGSIVEO was 20.6 months (range: 0.3 to 33.6).
Serious adverse reactions occurred in 20% of patients who received OGSIVEO. Serious adverse reactions occurring in ≥ 2% of patients were ovarian toxicity (4%).
Permanent discontinuation of OGSIVEO due to an adverse reaction occurred in 20% of patients. Adverse reactions which resulted in permanent discontinuation of OGSIVEO in ≥ 2% of patients were diarrhea, ovarian toxicity, increased ALT, and increased AST.
Dosage interruptions of OGSIVEO due to an adverse reaction occurred in 51% of patients. Adverse reactions which required dosage interruption in ≥ 2% of patients included diarrhea, rash, stomatitis, hypophosphatemia, fatigue, folliculitis, nausea, and ovarian toxicity.
Dose reductions of OGSIVEO due to an adverse reaction occurred in 42% of patients. Adverse reactions which required dose reductions in ≥ 2% of patients included diarrhea, rash, stomatitis, hypophosphatemia, folliculitis, hidradenitis, and ovarian toxicity.
The most common (≥ 15% with a difference between arms of ≥ 5% compared to placebo) adverse reactions that occurred in patients receiving OGSIVEO were diarrhea, ovarian toxicity, rash, nausea, fatigue, stomatitis, headache, abdominal pain, cough, alopecia, upper respiratory tract infection and dyspnea.
Table 2 summarizes the adverse reactions that occurred in DeFi.
Table 2. Adverse Reactions (≥ 15%) in Patients with Desmoid Tumor Who Received OGSIVEO with a Difference Between Arms of ≥ 5% Compared to Placebo on DeFi Adverse Reaction OGSIVEO
(N = 69)Placebo
(N = 72)All Grades
(%)Grade 3
(%)All Grades
(%)Grade 3
(%)Gastrointestinal Diarrhea 84 16 35 1.4 Nausea 54 1.4 39 0 Stomatitis a 39 4 4 0 Abdominal Pain a 22 1.4 14 1.4 Reproductive System Ovarian toxicity a,b 75c 0 0 0 Skin and Subcutaneous Tissue Rash a 68 6 14 0 Alopecia 19 0 1.4 0 General Fatigue a 54 2.9 38 0 Nervous System Headache a 30 0 15 0 Respiratory Cough a 20 0 6 0 Dyspnea 16 0 6 0 Infections Upper respiratory tract infection a 17 0 2.8 0 a Includes multiple related composite terms.
b Investigator assessment of ovarian toxicity included ovarian failure, premature menopause, amenorrhea, and menopause
c The number of females of reproductive potential in each arm is used as the denominator (OGSIVEO N = 36, Placebo N = 37)
Clinically relevant adverse reactions occurring in < 15% of patients receiving OGSIVEO in DeFi included non-melanoma skin cancers, epistaxis, hidradenitis suppurativa, folliculitis, and influenza-like illness.
Table 3 summarizes laboratory abnormalities in DeFi.
Table 3. Laboratory Abnormalities (≥15%) that Worsened from Baseline in Patients with Desmoid Tumor Who Received OGSIVEO in DeFi Laboratory Abnormality OGSIVEO Placebo All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)Chemistry Decreased phosphate a,b 65 Not Applicable 11 Not Applicable Increased urine glucose c,d 51 Not Applicable 0 Not Applicable Increased urine protein c 40 0 25 0 Increased aspartate aminotransferase a 33 2.9 18 1.4 Increased alanine aminotransferase a 30 6 21 1.4 Decreased potassium a 22 1.4 4.2 0 a The denominator used to calculate the rate was 69 for nirogacestat and 72 for placebo based on the number of patients with a baseline value and at least one post-treatment value.
b CTCAE Version 5.0 does not include numeric thresholds for grading of hypophosphatemia; all grades represent patients with lab value < Lower Limit of Normal (LLN).
c The denominator used to calculate the rate was 68 for nirogacestat and 69 for placebo based on the number of patients with a baseline value and at least one post-treatment value.
d CTCAE Version 5.0 does not include numeric thresholds for grading of increased urine glucose.
- Diarrhea [see Warnings and Precautions (5.1)]
-
7
DRUG INTERACTIONS
7.1 Effects of Other Drugs on OGSIVEO
Table 4. Effects of Other Drugs on OGSIVEO Strong or Moderate CYP3A Inhibitors Clinical Effect Nirogacestat is a CYP3A substrate. Strong or moderate CYP3A inhibitors increase nirogacestat exposure [see Clinical Pharmacology (12.3)], which may increase the risk of OGSIVEO adverse reactions. Prevention or Management Avoid concomitant use of OGSIVEO with strong or moderate CYP3A inhibitors including grapefruit products, Seville oranges, and starfruit. Strong or Moderate CYP3A Inducers Clinical Effect Nirogacestat is a CYP3A substrate. Strong or moderate CYP3A inducers decrease serum nirogacestat exposure [see Clinical Pharmacology (12.3)], which may reduce the effectiveness of OGSIVEO. Prevention or Management Avoid concomitant use of OGSIVEO with strong or moderate CYP3A inducers. Gastric Acid Reducing Agents Clinical Effect Nirogacestat is poorly soluble at pH ≥ 6. Gastric acid reducing agents may decrease serum nirogacestat exposure [see Clinical Pharmacology (12.3)], which may reduce the effectiveness of OGSIVEO. Prevention or Management Avoid concomitant use with proton pump inhibitors and H2 blockers.
If concomitant use cannot be avoided, OGSIVEO can be staggered with antacids (e.g., administer OGSIVEO 2 hours before or 2 hours after antacid use).7.2 Effects of OGSIVEO on Other Drugs
Table 5. Effects of OGSIVEO on Other Drugs Certain CYP3ASubstrates Clinical Effect Nirogacestat increases exposure of CYP3A substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to these substrates. Prevention or Management Avoid concomitant use with CYP3A substrates where minimal concentration changes may lead to serious adverse reactions. Certain CYP2C19 Substrates Clinical Effect Nirogacestat decreases exposure of CYP2C19 substrates [see Clinical Pharmacology (12.3)], which may decrease efficacy of these substrates. Prevention or Management Avoid concomitant use with OGSIVEO where decreased concentrations of CYP2C19 substrates may lead to significant decreases in efficacy of the CYP2C19 substrate unless otherwise recommended in the Prescribing Information for the CYP2C19 substrate. -
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action, OGSIVEO can cause fetal harm or loss of pregnancy when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. Oral administration of nirogacestat to pregnant rats during the period of organogenesis resulted in embryo-fetal toxicity and embryo-fetal death at maternal exposures below the human exposure at the recommended dose of 150 mg twice daily [see Data]. There are no available data on the use of OGSIVEO in pregnant women. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Daily oral administration of nirogacestat to pregnant rats during the period of organogenesis resulted in decreased fetal body weights, pre- and post-implantation loss, and fetal subcutis edema at doses ≥ 20 mg/kg/day (approximately 0.85 times the recommended dose of 150 mg twice daily based on area under the curve).
8.2 Lactation
Risk Summary
There are no data on the presence of nirogacestat or its metabolites in human milk or the effects of nirogacestat on a breastfed child or milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with OGSIVEO and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
OGSIVEO can cause fetal harm when administered to a pregnant woman (see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating OGSIVEO [see Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with OGSIVEO and for 1 week after the last dose.
Males
Advise males with female partners of reproductive potential to use effective contraception during treatment with OGSIVEO and for 1 week after the last dose.
Infertility
Based on findings in animal studies, OGSIVEO can impair female and male fertility. OGSIVEO has been shown to interfere with folliculogenesis and spermatogenesis in nonclinical studies resulting in changes that included ovarian atrophy [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of OGSIVEO have not been established in pediatric patients. Epiphyseal disorder, manifesting as a widening of the epiphyseal growth plate, has been reported in pediatric patients with open growth plates treated with OGSIVEO.
8.5 Geriatric Use
Of the total number of OGSIVEO-treated patients in the DeFi study, 3 (4%) were 65 years of age and older and none were 75 years of age and older. Clinical studies of OGSIVEO did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently than younger adult patients.
-
10
OVERDOSAGE
Due to the high level of protein binding, OGSIVEO is not expected to be dialyzable [see Clinical Pharmacology (12.3)].
-
11
DESCRIPTION
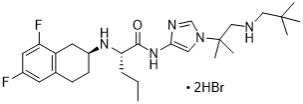
OGSIVEO oral tablets contain nirogacestat (as nirogacestat hydrobromide), a gamma (ɣ) secretase inhibitor. Nirogacestat hydrobromide is chemically known as (S)-2-(((S)-6,8-Difluoro-1,2,3,4-tetrahydronaphthalen-2-yl)amino)-N-(1-(2-methyl-1-(neopentylamino)propan-2-yl)-1H-imidazol-4-yl) pentanamide dihydrobromide. The empirical formula is C27H43Br2F2N5O and the molecular weight is 651.48 g/mol. Nirogacestat hydrobromide is a white to off white powder with an aqueous solubility of 11.4 mg/mL and a pH of 4.4 in water at 25°C. Nirogacestat dihydrobromide is highly soluble at low pH, however the solubility significantly decreases at pH > 6.0. The molecule has pKa’s of 5.77 and 7.13. The structural formula for nirogacestat hydrobromide is:
OGSIVEO (nirogacestat) tablets are immediate release (IR), film-coated tablets intended for oral administration. Each 50 mg tablet contains 50 mg nirogacestat as 66.525 mg nirogacestat hydrobromide. OGSIVEO 50 mg tablets are round, biconvex with an approximate diameter of 8 mm. They are film coated, orange in color, and debossed with “50” on one face and plain on the other face. Each 100 mg tablet contains 100 mg nirogacestat as 133.050 mg nirogacestat hydrobromide. OGSIVEO 100 mg tablets are round, biconvex with an approximate diameter of 10 mm. They are film coated, light orange in color, and debossed with “100” on one face and plain on the other face. Each 150 mg tablet contains 150 mg nirogacestat as 199.574 mg nirogacestat hydrobromide. OGSIVEO 150 mg tablets are oval, biconvex with approximate dimensions of 8.5 X 17.5mm. They are film coated, orange yellow in color, and debossed with “150” on one face and plain on the other face.
OGSIVEO tablets contain the following inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate type A. The tablets are finished with Opadry® QX orange film coating consisting of the following ingredients: FD&C yellow #6/sunset yellow FCF aluminum lake, glycerol monocaprylocaprate type 1/mono/diglycerides, iron oxide yellow, macrogol (PEG) polyvinyl alcohol graft copolymer, polyvinyl alcohol – partially hydrolyzed, talc, and titanium dioxide.
-
12
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nirogacestat is a gamma secretase inhibitor that blocks proteolytic activation of the Notch receptor. When dysregulated, Notch can activate pathways that contribute to tumor growth.
12.2 Pharmacodynamics
Exposure-Response Relationships
There is an exposure-response relationship between nirogacestat exposure and Grade 3 hypophosphatemia with a higher risk of Grade 3 hypophosphatemia at higher exposure.
Cardiac Electrophysiology
At the recommended dosage, a mean increase in the QTc interval > 20 ms was not observed.
12.3 Pharmacokinetics
Nirogacestat pharmacokinetic parameters in patients with desmoid tumors are summarized in Table 6.
Table 6. Pharmacokinetic Parameters and Characteristics of Nirogacestat General Information Steady state exposure
[Mean (%CV)]Cmax 508 (62) ng/mL AUC0-tau 3370 (58) ng·h/mL Time to steady-state Approximately 6 days Accumulation ratio [Median (Min, Max)] 1.6 (1.3, 4.6) Absorption Tmax [Median (Min, Max)] 1.5 (0.5, 6.5) hours Absolute bioavailability 19% Food effect [dose-normalized GMR% (90% CI)] Cmax 93 % (55%, 166%) AUC 114% (76%, 171%) Distribution Serum protein binding 99.6% Protein Binding* Human serum albumin 94.6% α-1 acid glycoprotein 97.9% Apparent volume of distribution (Vz/F)
[Mean (%CV)]1430 (65) L Elimination Apparent Systemic Clearance (CL/F)
[Mean (%CV)]45 (58) L/hr Terminal elimination half-life (t1/2)
[Mean (%CV)]23 (37) hr Metabolism Primary pathway N-dealkylation via CYP3A4 (85%)
Secondary pathways Metabolism by CYP 3A4, 2C19, 2C9, and 2D6 Excretion Feces 38% Urine 17% (<1% unchanged) Expired air 9.7% * Protein binding values reflect results from separate assays.
Abbreviations: AUC0-tau = area under the time concentration curve to the dosing interval;
Cmax = maximum plasma concentration; Tmax = time to reach Cmax; GMR = geometric mean ratio
Specific Populations
No clinically significant differences in the pharmacokinetics of nirogacestat were observed based on age (18 to 80 years), sex, race (Asian, Black or African American, and White), or mild or moderate renal impairment (eGFR ≥41 mL/min/1.73m2).
Effects of Hepatic Impairment
The mean AUC increased by up to 16% and the mean Cmax decreased by up to 39% in subjects with moderate hepatic impairment identified by Child-Pugh Class B or NCI-ODWG Group C criteria compared to that of subjects with normal hepatic function.
Insufficient data are available to characterize nirogacestat PK in patients with severe hepatic impairment.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Strong CYP3A inhibitors: Nirogacestat Cmax increased by 2.5-fold and AUC by 8.2-fold following coadministration of a single dose of OGSIVEO (100 mg) with itraconazole (a strong CYP3A inhibitor). Nirogacestat AUC is predicted to increase by 6.33-, 5.19-, and 3.46-fold following coadministration of multiple doses of OGSIVEO (150 mg BID) with itraconazole, ketoconazole and clarithromycin (strong CYP3A inhibitors), respectively.
Moderate CYP3A inhibitors: Nirogacestat AUC is predicted to increase by 2.73- and 3.18-fold following coadministration of multiple doses of OGSIVEO (150 mg BID) with erythromycin (moderate CYP3A inhibitor) and fluconazole (moderate CYP3A inhibitor), respectively.
Strong CYP3A inducers: Nirogacestat AUC is predicted to decrease by 85% following coadministration of multiple doses of OGSIVEO (150 mg BID) with rifampin (strong CYP3A inducer).
Moderate CYP3A inducers: Nirogacestat AUC is predicted to decrease by 67% following coadministration of multiple doses of OGSIVEO (150 mg BID) with efavirenz (moderate CYP3A inducer).
CYP3A substrates: Midazolam (CYP3A substrate) Cmax is predicted to increase by 1.77-fold and AUC by 2.07-fold following concomitant use with multiple doses of OGSIVEO (150 mg BID).
CYP2C19 substrates: Concomitant use of multiple doses of OGSIVEO (150 mg BID) with a drug that is a sensitive substrate of CYP2C19 decreases the plasma concentrations of these substrates.
P-gp substrates: Exposure to dabigatran (P-gp substrate) Cmax and AUC were not affected by concomitant use with nirogacestat.
Drugs that increase gastric pH: Concomitant administration of proton pump inhibitors (e.g., omeprazole), histamine type 2 (H2)-receptor antagonists (e.g., famotidine), or antacids (e.g., calcium) is expected to reduce concentrations of nirogacestat.
Other Drugs:
No clinically significant differences in nirogacestat pharmacokinetics were predicted when used concomitantly with cimetidine (weak CYP3A inhibitor).
No clinically significant differences were predicted in the pharmacokinetics of the following drugs when used concomitantly with nirogacestat: rosiglitazone (CYP2C8 substrate) or
S-warfarin (CYP2C9 substrate).In Vitro Studies
Nirogacestat does not inhibit CYP 1A2, 2B6, 2C8, 2C9, 2C19, and 2D6.
Nirogacestat is an inducer of CYP2B6, CYP2C8, CYP2C9, and CYP2C19, but does not induce CYP 1A2.
Transporter systems: Nirogacestat is a P-gp substrate, but not a substrate of BCRP, OATP1B1, and OATP1B3.
Nirogacestat is a P-gp inhibitor, but not an inhibitor of BCRP, MATE1, MATE2-K, OATP1B1, OATP1B3, OAT1, OAT2 and OAT3.
-
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 6-month carcinogenicity study, transgenic rasH2 mice received up to 100 mg/kg/day of oral nirogacestat, resulting in mean exposure levels (AUC) less than those in humans at the recommended dose of 150 mg twice daily. No statistically significant neoplastic findings occurred. The carcinogenic potential of nirogacestat in rats has not been assessed.
Mutagenesis
Nirogacestat was not mutagenic in a bacterial reverse mutation (Ames) assay and was not clastogenic in an in vitro chromosome aberration assay in human lymphocytes or in vivo rat bone marrow micronucleus study.
Impairment of Fertility
Nirogacestat resulted in reduced fertility when administered to male and female rats at doses ≥ 5 mg/kg/day (approximately 0.16 times the recommended dose of 150 mg twice daily based on body surface area (BSA), and a lack of fertility when administered to male and female rats at doses ≥ 40 mg/kg/day (approximately 1.3 times the recommended dose of 150 mg twice daily based on BSA). Adverse findings in rats included ovarian atrophy, reduced testes weights, and decreased sperm concentration and motility.
-
14
CLINICAL STUDIES
14.1 Desmoid Tumor
The efficacy of OGSIVEO was evaluated in DeFi (NCT03785964), an international, multicenter, randomized (1:1), double-blind, placebo-controlled trial in 142 adult patients with progressing desmoid tumors not amenable to surgery. Patients were eligible if the desmoid tumor had progressed within 12 months of screening. Patients with progressing desmoid tumor that would result in immediate risk to the patient were not eligible.
Patients were randomized to receive 150 mg OGSIVEO or placebo orally twice daily until disease progression or unacceptable toxicity. Patients were stratified by primary tumor(s) location (intra-abdominal versus extra-abdominal). Tumor imaging occurred every 3 months. Crossover was permitted at the time of radiographic progression. The major efficacy outcome was progression-free survival (PFS) based on RECIST v1.1 as assessed by blinded independent central review or on clinical progression by the investigator (and confirmed by independent review). Clinical progression required worsening of symptoms resulting in a global deterioration of health status causing the permanent discontinuation from trial treatment and the initiation of emergent treatment (e.g., radiotherapy, surgery, or systemic therapy including chemotherapy or tyrosine kinase inhibitors) for desmoid tumors. Objective response rate (ORR) was an additional efficacy outcome measure. Worst pain (item 3) was assessed daily using Brief Pain Inventory-Short Form (BPI-SF), an 11-point numerical rating scale ranging from 0 (“no pain”) to 10 (“pain as bad as you can imagine”) and averaged over 7 days prior to each visit.
A total of 142 patients were randomized. The median age was 34 years (range: 18 to 76); 65% were female; race was 83% White, 6% Black, 3% Asian, and other or not reported in 8%; and 73% had an ECOG performance status (PS) of 0, 27% had an ECOG PS of 1, and 0.7% had an ECOG PS of 2. Twenty-three percent of patients had intra-abdominal disease or both intra- and extra-abdominal disease, and 77% had only extra-abdominal disease. Forty-one percent of patients had multifocal disease and 59% had single focal disease. Of 105 patients with known tumor mutation status, 81% had a CTNNB1 mutation and 21% had an APC mutation. Seventeen percent of patients had a family history of familial adenomatous polyposis (FAP). Twenty-three percent of patients received no prior therapy, and 44% received ≥3 prior lines of therapy. Prior therapy included surgery (53%), radiotherapy (23%), and systemic therapy (61%). Thirty-three percent of patients were previously treated with a tyrosine kinase inhibitor and 36% were previously treated with chemotherapy. Fifty percent had a BPI-SF item 3 (worst pain) score of ≥2.
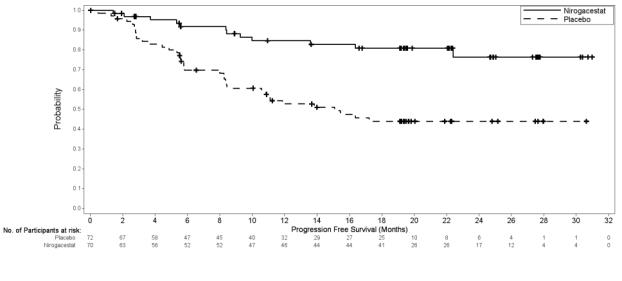
Efficacy results are summarized in Table 7 and Figure 1.
Table 7. Efficacy Results of DeFi OGSIVEO
N = 70Placebo
N = 72Progression-free Survival Number (%) of patients with event 12 (17) 37 (51) Radiographic progression a 11 (16) 30 (42) Clinical progression a 1 (1) 6 (8) Death 0 1 (1) Median (months) (95% CI) b NR (NR, NR) 15.1 (8.4, NR) Hazard ratio (95% CI) 0.29 (0.15, 0.55) p-value c < 0.001 Objective Response Rate a ORR, n (%)
95% CI d29 (41)
(29.8, 53.8)6 (8)
(3.1, 17.3)CR 5 (7) 0 PR 24 (34) 6 (8) p-value e <0.001 Abbreviations: CI: confidence interval; CR: complete response; ORR: objective response rate; PR: partial response; NR: Not Reached
a Assessed by blinded independent central review.
b Obtained using Kaplan-Meier Methodology.
c p-value was from a one-sided stratified log-rank test with placebo as reference.
d Obtained using exact method based on binomial distribution.
e p-value was from a two-sided Cochran-Mantel-Haenszel test.
Figure 1. Kaplan-Meier Curve of PFS in DeFi
PFS results were supported by change from baseline in patient-reported worst pain favoring the OGSIVEO arm.
An exploratory analysis of PFS based on only radiographic progression demonstrated a hazard ratio of 0.31 (95% CI: 0.16, 0.62).
-
16
HOW SUPPLIED/STORAGE AND HANDLING
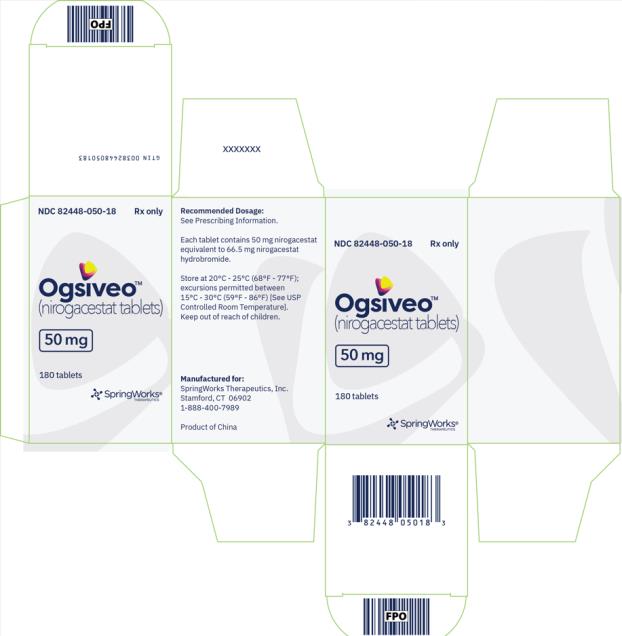
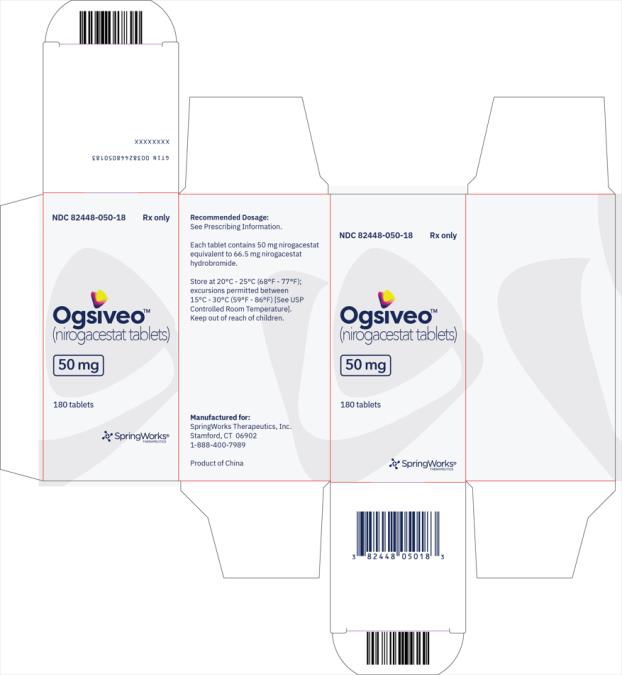
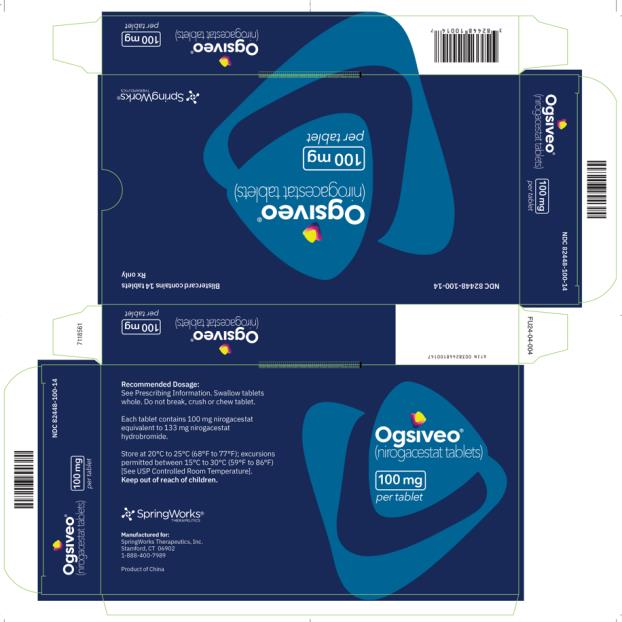
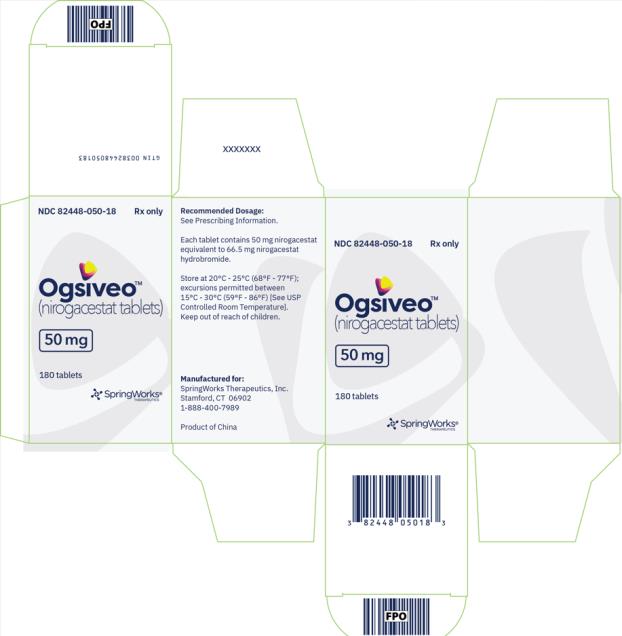
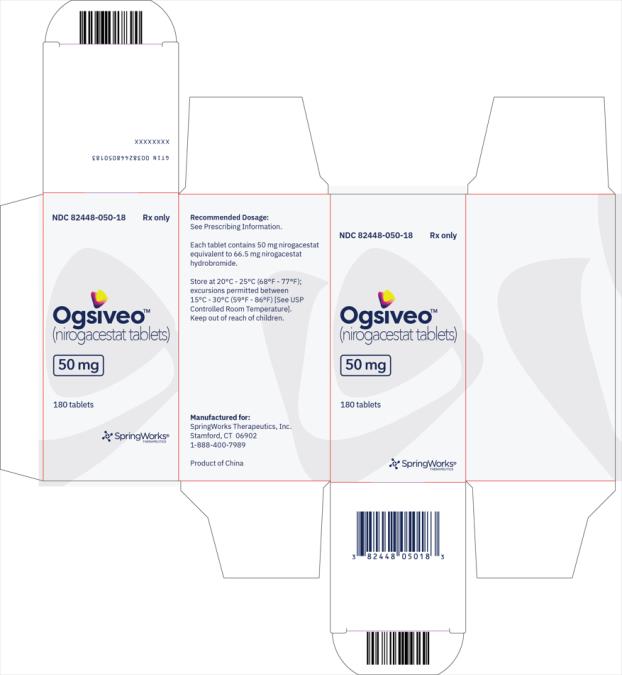
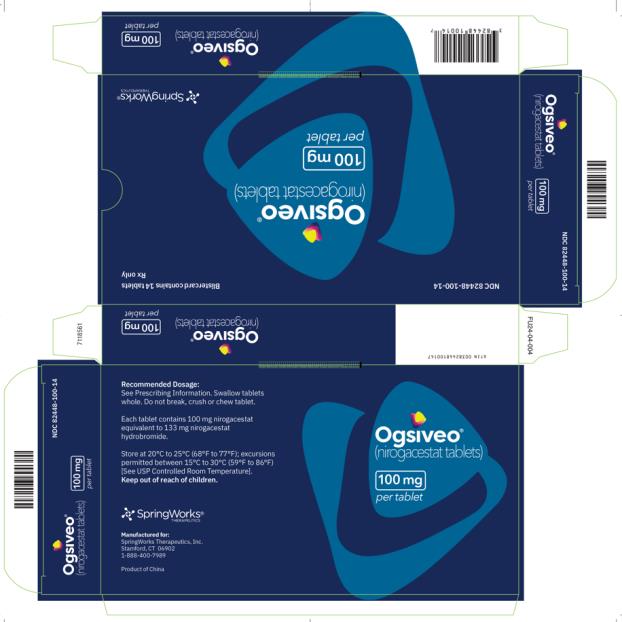
OGSIVEO (nirogacestat) is supplied as 50 mg, 100 mg, and 150 mg tablets. Each 50 mg orange, film-coated tablet is debossed with a “50” on one side. Each 100 mg light orange, film-coated tablet is debossed with a “100” on one face. Each 150 mg orange yellow, film-coated tablet is debossed with a “150” on one face.
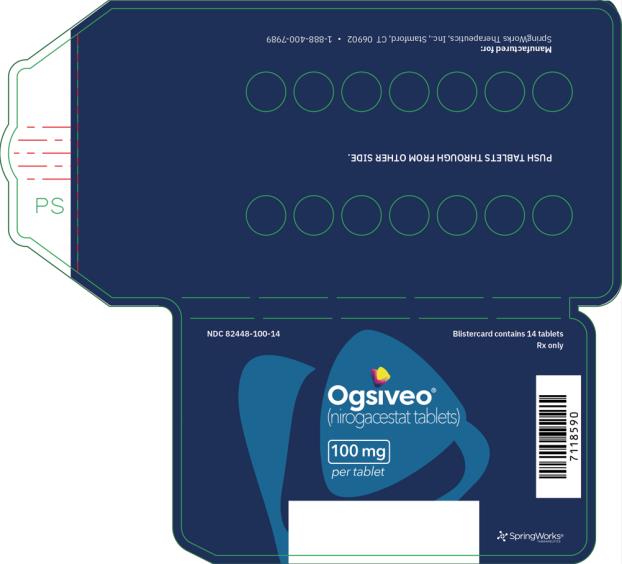
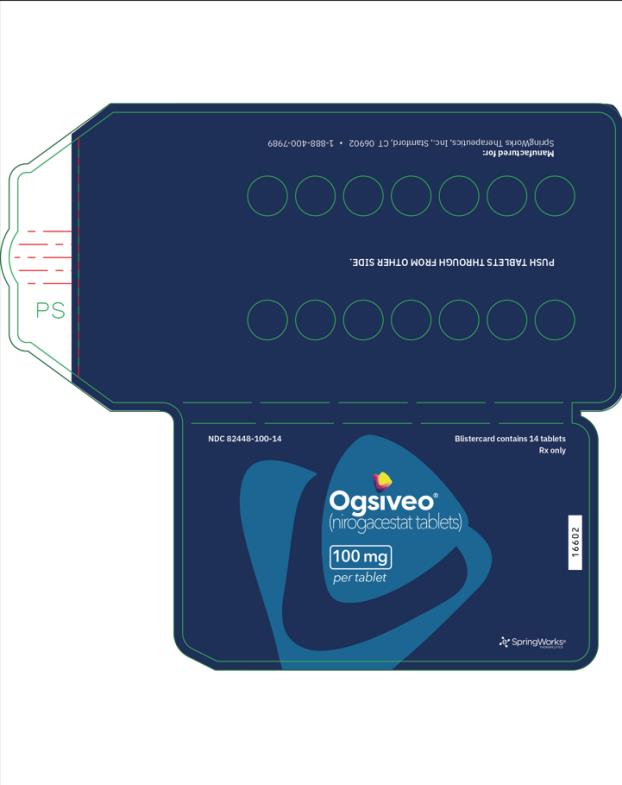
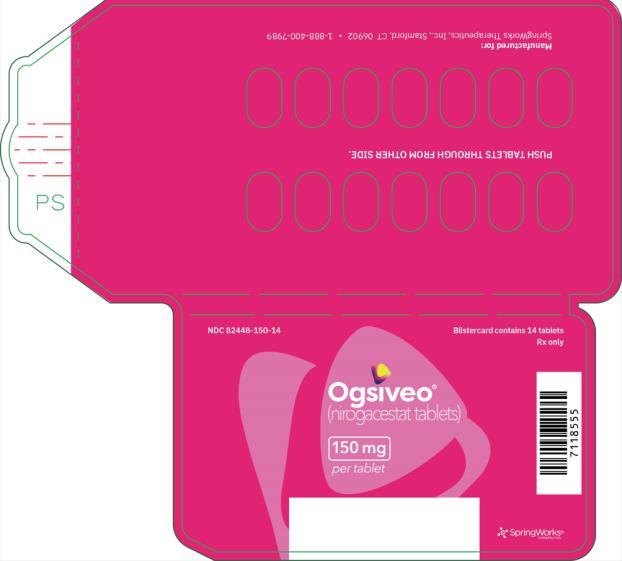
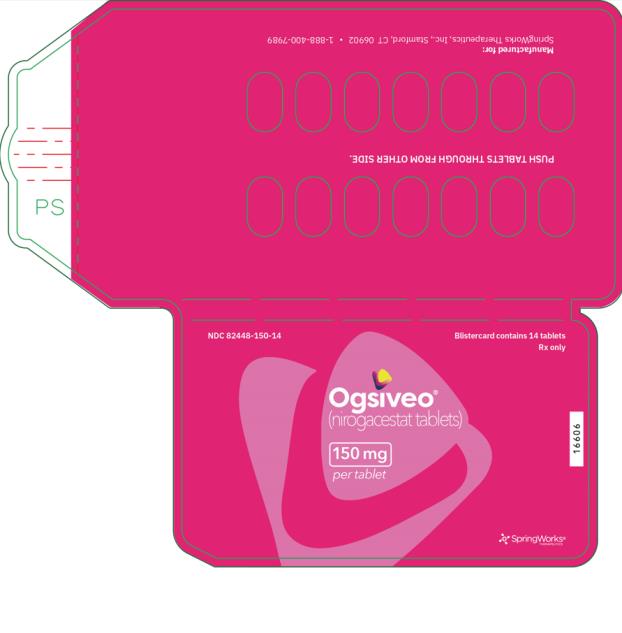
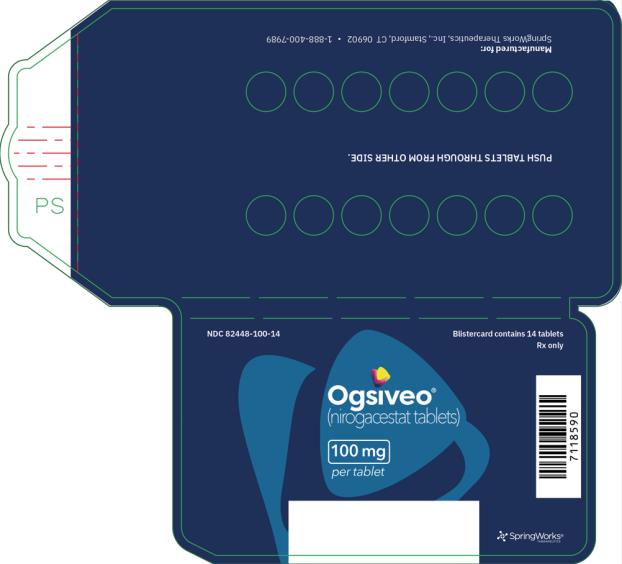
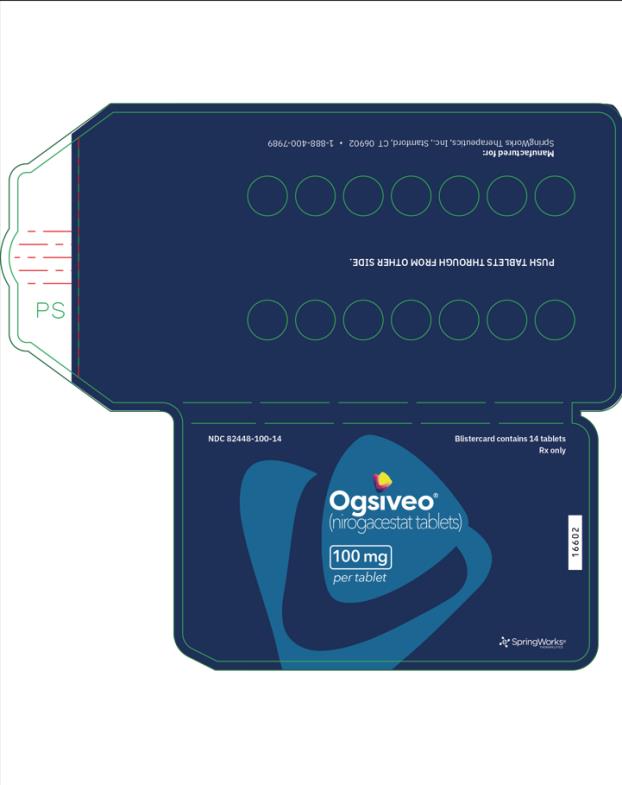
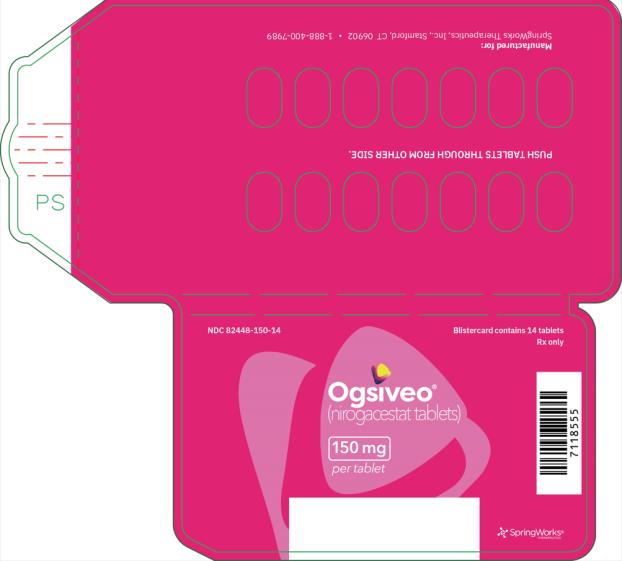
The 50 mg tablets are packaged in high density polyethylene (HDPE) bottles with child-resistant closures. Each bottle contains 180 tablets: NDC # 82448-050-18. The 100 and 150 mg tablets are packaged in 14 count blister cards with child resistant closure (100 mg - NDC #82448-100-14; 150 mg - NDC #82448-150-14).
Store at 20°C-25°C (68°F-77°F). Excursions permitted between 15°C-30°C (59°F-86°F). See USP Controlled Room Temperature.
-
17
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Diarrhea
Advise patients that OGSIVEO can cause diarrhea, which may be severe, and to contact their healthcare provider for sustained diarrhea that does not respond to supportive care [see Dosage and Administration (2.2),Warnings and Precautions (5.1)].
Ovarian Toxicity
Advise females of reproductive potential that OGSIVEO can cause ovarian toxicity and impair fertility, and that these effects may continue following discontinuation of OGSIVEO. Advise patients to tell their healthcare provider if they experience symptoms of ovarian toxicity, including hot flashes or menstrual irregularities [see Warnings and Precautions (5.2)].
Liver Toxicity
Advise patients that OGSIVEO can cause ALT or AST elevations, and that their healthcare provider should monitor liver transaminase levels regularly [see Dosage and Administration (2.2), Warnings and Precautions (5.3)].
Non-Melanoma Skin Cancers
Advise patients that OGSIVEO can cause new non-melanoma skin cancers, that they will be monitored for these, and to contact their healthcare provider for any new or changing lesions on their skin [see Warnings and Precautions (5.4)].
Electrolyte Abnormalities
Advise patients that OGSIVEO can cause hypophosphatemia and/or hypokalemia which may require phosphate and/or potassium supplementation. Advise patients that they will be monitored for these and to contact their healthcare provider if they experience muscle pain or weakness [see Dosage and Administration (2.2), Warnings and Precautions (5.5)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential harm to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy, and to stop taking OGSIVEO if they become pregnant. Also, advise females of reproductive potential to use effective contraception during treatment with OGSIVEO and for 1 week after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with OGSIVEO and for 1 week after the last dose [see Warnings and Precautions (5.6), Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with OGSIVEO and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Drug Interactions
Advise patients to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products. Inform patients to avoid starfruit, Seville oranges, grapefruit, and juice from any of these fruits when taking OGSIVEO [see Drug Interactions (7)].
Manufactured for:
SpringWorks Therapeutics, Inc. Stamford, CT 06902
OGSIVEO® is a trademark of SpringWorks Therapeutics, Inc.
©2024 SpringWorks Therapeutics, Inc.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
OGSIVEO (og-SIH-vee-oh)
(nirogacestat)
tabletsWhat is OGSIVEO?
OGSIVEO is a prescription medication used to treat adults with progressing desmoid tumors who require a medicine by mouth or injection (systemic therapy).
It is not known if OGSIVEO is safe and effective in children.Before taking OGSIVEO tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems.
- are pregnant or plan to become pregnant. OGSIVEO can harm your unborn baby. Tell your healthcare provider if you become pregnant or think you may be pregnant during treatment with OGSIVEO.
- Your healthcare provider will give you a pregnancy test before you start treatment with OGSIVEO.
- You should use effective birth control (contraception) during treatment with OGSIVEO and for 1 week after the last dose. Talk to your healthcare provider about birth control methods that may be right for you.
- Stop taking OGSIVEO and tell your healthcare provider right away if you become pregnant.
- Males with female partners who are able to become pregnant should use effective birth control (contraception) during treatment with OGSIVEO and for 1 week after the last dose.
- are breastfeeding or plan to breastfeed. It is not known if OGSIVEO passes into your breast milk. Do not breastfeed during treatment with OGSIVEO and for 1 week after the last dose.
You should avoid taking proton pump inhibitors (PPIs) and H2 blockers during treatment with OGSIVEO. Ask your healthcare provider if you are not sure if you take one of these medicines.How should I take OGSIVEO? - Take OGSIVEO exactly as your healthcare provider tells you to take it.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with OGSIVEO if you develop side effects.
- Take OGSIVEO 2 times a day with or without food.
- Swallow OGSIVEO tablets whole. Do not break, crush or chew tablets.
- If you take an antacid medicine, take OGSIVEO 2 hours before or 2 hours after taking the antacid.
- If you vomit after taking a dose or miss a dose of OGSIVEO, take your next dose at your regular time. Do not take 2 doses of OGSIVEO to make up the dose.
What should I avoid while taking OGSIVEO?
Avoid eating or drinking grapefruit products, Seville oranges, and starfruit during treatment with OGSIVEO.What are the possible side effects of OGSIVEO?
OGSIVEO can cause serious side effects, including:
-
Diarrhea. Diarrhea is common during treatment with OGSIVEO and may sometimes be severe. Your healthcare provider may tell you to drink more fluids or may tell you to take antidiarrheal medicines. Tell your healthcare provider right away if you have diarrhea that lasts longer than a few days and does not get better after taking antidiarrheal medicines.
-
Ovarian problems. Females who are able to become pregnant may have ovarian problems and changes in their menstrual cycle during treatment with OGSIVEO. OGSIVEO may affect fertility which may affect your ability to have a child. Tell your healthcare provider if you have any changes in your menstrual cycle or any hot flashes, night sweats or vaginal dryness during treatment with OGSIVEO.
-
Liver problems. OGSIVEO can cause an increase in liver enzymes. Your healthcare provider will do blood tests to check your liver function before you start and during your treatment with OGSIVEO.
-
New Non-Melanoma Skin Cancers. Your healthcare provider will do skin exams before and during treatment with OGSIVEO if you are at risk for skin cancer. Tell your healthcare provider if you have any new or changing skin lesions.
- Electrolyte (salt) problems. Your healthcare provider will do blood tests to check your phosphate and potassium levels during treatment with OGSIVEO and may give you medicines to treat low phosphate or low potassium if needed. Tell your healthcare provider if you develop any muscle pain or weakness.
- rash
- stomach (abdominal) pain
- nausea
- cough
- tiredness
- hair loss
- mouth sores
- upper respiratory infection
- headache
- shortness of breath
OGSIVEO can affect fertility in females and males, which may affect your ability to have a child. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of OGSIVEO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How do I store OGSIVEO?
- Store OGSIVEO tablets at room temperature between 68°F to 77°F (20°C to 25°C).
General information about safe and effective use of OGSIVEO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use OGSIVEO for a condition for which it is not prescribed. Do not give OGSIVEO to other people even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about OGSIVEO that is written for health professionals.What are the ingredients in OGSIVEO?
Active ingredient: nirogacestat
Inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate type A.
Film Coating ingredients: FD&C yellow #6/sunset yellow FCF aluminum lake, glycerol monocaprylocaprate type 1/mono/diglycerides, iron oxide yellow, macrogol (PEG) polyvinyl alcohol graft copolymer, polyvinyl alcohol – partially hydrolyzed, talc, and titanium dioxide.
Manufactured for SpringWorks Therapeutics, Inc.
Stamford, CT 06902.
OGSIVEO® is a trademark of SpringWorks Therapeutics, Inc.
©2024 SpringWorks Therapeutics, Inc. - have liver problems.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OGSIVEO
nirogacestat tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82448-050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIROGACESTAT (UNII: QZ62892OFJ) (NIROGACESTAT - UNII:QZ62892OFJ) NIROGACESTAT 50 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) GLYCERYL MONOCAPRYLOCAPRATE (UNII: G7515SW10N) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color orange Score no score Shape ROUND Size 8mm Flavor Imprint Code 50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82448-050-18 180 in 1 BOTTLE; Type 0: Not a Combination Product 11/27/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217677 11/27/2023 OGSIVEO
nirogacestat tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82448-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIROGACESTAT (UNII: QZ62892OFJ) (NIROGACESTAT - UNII:QZ62892OFJ) NIROGACESTAT 100 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) GLYCERYL MONOCAPRYLOCAPRATE (UNII: G7515SW10N) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color orange (light orange) Score no score Shape ROUND Size 10mm Flavor Imprint Code 100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82448-100-14 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 11/27/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217677 11/27/2023 OGSIVEO
nirogacestat tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82448-150 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIROGACESTAT (UNII: QZ62892OFJ) (NIROGACESTAT - UNII:QZ62892OFJ) NIROGACESTAT 150 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) GLYCERYL MONOCAPRYLOCAPRATE (UNII: G7515SW10N) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color orange (orange yellow) Score no score Shape ROUND Size 18mm Flavor Imprint Code 150 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82448-150-14 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 11/27/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217677 11/27/2023 Labeler - SpringWorks Therapeutics, Inc. (116976800)