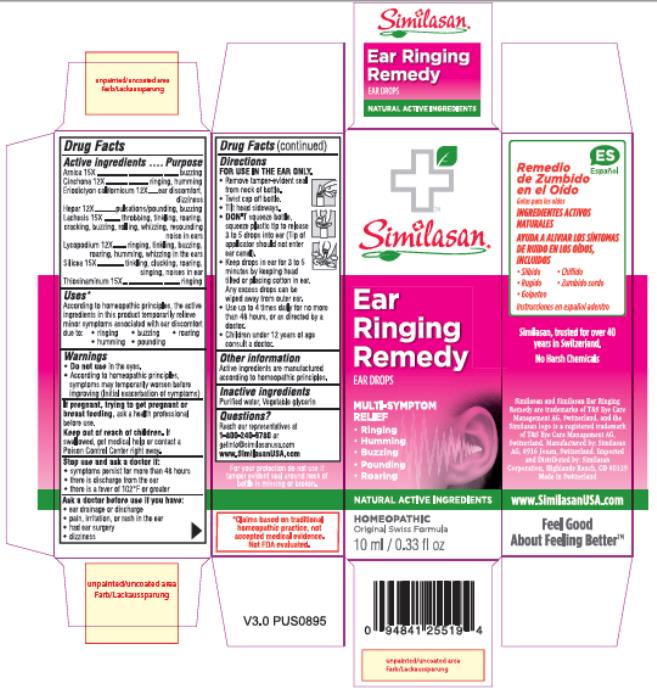
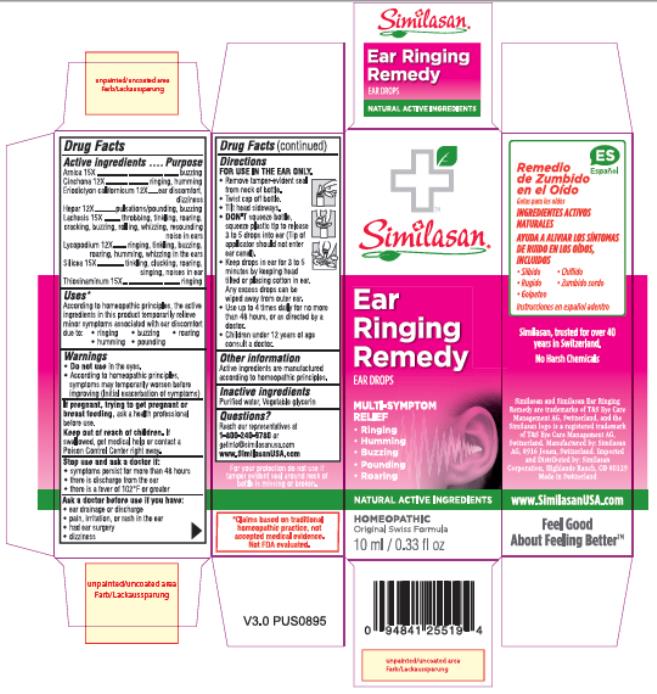
Label: EAR RINGING REMEDY- allylthiourea, arnica montana flower, calcium sulfide, eriodictyon californicum leaf, lachesis muta venom, lycopodium clavatum spore, quinine sulfate, silica dimethyl silylate solution
- NDC Code(s): 53799-275-11
- Packager: Similasan AG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 11, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses*
-
Warnings
-
Do not use in the eyes.
- According to homeopathic principles, symptoms may temporarily worsen before improving (Initial exacerbation of symptoms)
If pregnant, trying to get pregnant or breast feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
-
Do not use in the eyes.
-
Directions
FOR USE IN THE EAR ONLY.
- Remove tamper-evident seal from neck of bottle.
- Twist cap off bottle.
- Tilt head sideways.
-
DON'T squeeze bottle, squeeze plastic tip to release 3 to 5 drops into ear (Tip of applicator should not enter ear canal).
- Keep drops in ear for 3 to 5 minutes by keeping head tilted or placing cotton in ear. Any excess drops can be wiped away from outer ear.
- Use up to 4 times daily for no more than 48 hours, or as directed by a doctor.
- Children under 12 years of age consult a doctor.
- Remove tamper-evident seal from neck of bottle.
- Other information
- Inactive ingredient
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
EAR RINGING REMEDY
allylthiourea, arnica montana flower, calcium sulfide, eriodictyon californicum leaf, lachesis muta venom, lycopodium clavatum spore, quinine sulfate, silica dimethyl silylate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53799-275 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) (ARNICA MONTANA FLOWER - UNII:OZ0E5Y15PZ) ARNICA MONTANA FLOWER 15 [hp_X] in 10 mL QUININE SULFATE (UNII: KF7Z0E0Q2B) (QUININE - UNII:A7V27PHC7A) QUININE SULFATE 12 [hp_X] in 10 mL ERIODICTYON CALIFORNICUM LEAF (UNII: 2Y7TIQ135H) (ERIODICTYON CALIFORNICUM LEAF - UNII:2Y7TIQ135H) ERIODICTYON CALIFORNICUM LEAF 12 [hp_X] in 10 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 12 [hp_X] in 10 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 15 [hp_X] in 10 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 12 [hp_X] in 10 mL SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) (SILICA DIMETHYL SILYLATE - UNII:EU2PSP0G0W) SILICA DIMETHYL SILYLATE 15 [hp_X] in 10 mL ALLYLTHIOUREA (UNII: 706IDJ14B7) (ALLYLTHIOUREA - UNII:706IDJ14B7) ALLYLTHIOUREA 15 [hp_X] in 10 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53799-275-11 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 03/09/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/09/2021 Labeler - Similasan AG (481545754)