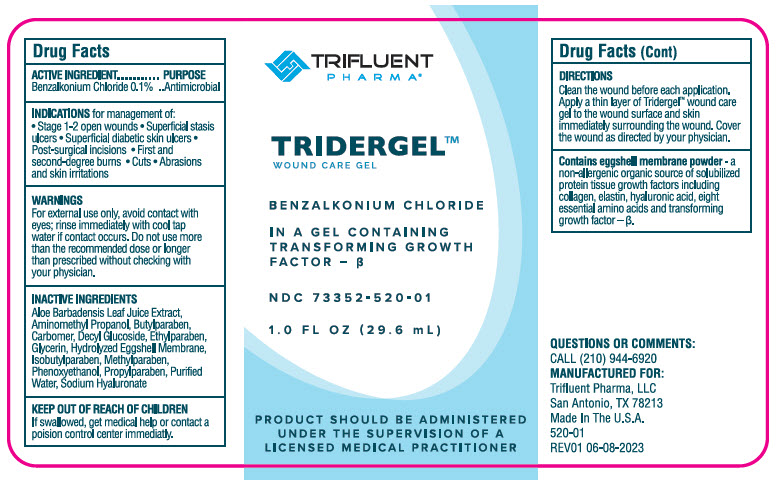
Label: TRIDERGEL- benzalkonium chloride gel
- NDC Code(s): 73352-520-01
- Packager: Trifluent Pharma LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- INDICATIONS & USAGE
-
Warnings
For external use only.
When using this product avoid contact with eyes; rinse immediately with cool tap water if contact occurs.
Do not use more than the recommended dose or longer than prescribed without checking with your physician. Talk with your physician before you use any other medicines or cleansers on your skin. Ask your physician before prolonged sun exposure. If your symptoms do not improve or they worsen, contact your physician
- Directions
- Inactive Ingredients
- QUESTIONS OR COMMENTS
- PRINCIPAL DISPLAY PANEL - 29.6 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
TRIDERGEL
benzalkonium chloride gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73352-520 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.001 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EGG SHELL MEMBRANE (UNII: N7QBR4212V) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) BUTYLPARABEN (UNII: 3QPI1U3FV8) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73352-520-01 29.6 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 12/11/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M005 12/11/2023 Labeler - Trifluent Pharma LLC (117167281)