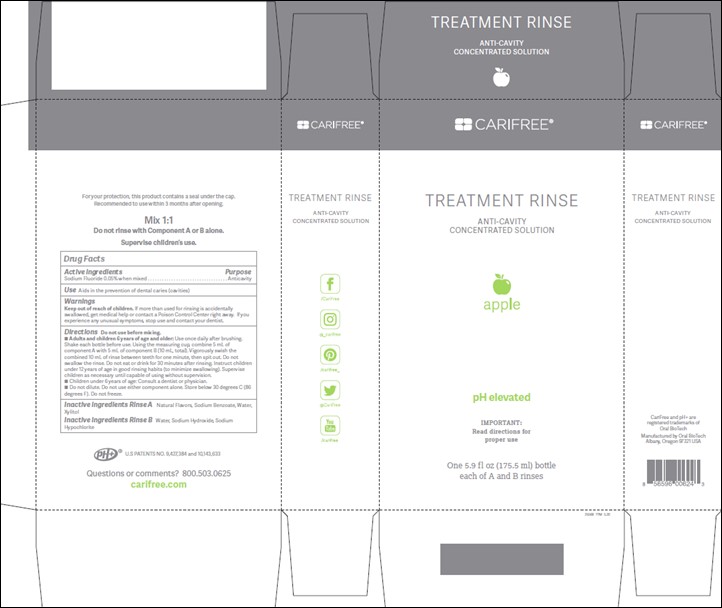
Label: TREATMENT RINSE APPLE- anticaries rinse
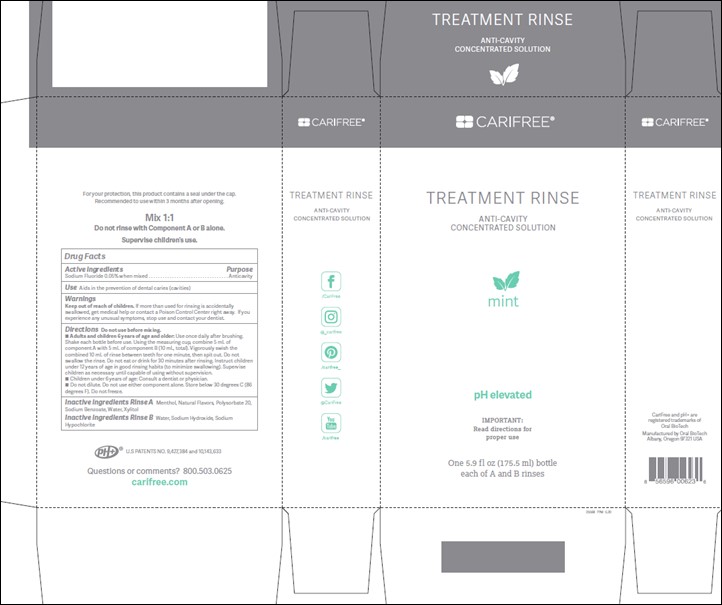
TREATMENT RINSE MINT- anticaries rinse
- NDC Code(s): 61578-314-01, 61578-315-01
- Packager: DENTAL ALLIANCE HOLDINGS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients:
- Purpose:
- Use:
- Warnings:
- Warnings:
-
Directions:
Do not use before mixing with Part B. Adults and children 6 years of age and older: Use once daily after brushing. Shake each bottle before use. Using the measuring cup, combine 5 mL of component A with 5 mL of component B (10 mL, total). Vigorously swish the combined 10 mL of rinse between teeth for one minute, then spit out. Do not swallow the rinse. Do not eat or drink for 30 minutes after rinsing. Instruct children under 12 years of age in good rinsing habits (to minimize swallowing). Supervise children as necessary until capable of using without supervision. Children under 6 years of age: Consult a dentist or physician. Do not dilute. Do not use either component alone. Store below 30 degrees C (86 degrees F). Do not freeze.
- Inactive ingredients:
- Treatment Rinse Mint and Apple enclosure and carton labels:
-
INGREDIENTS AND APPEARANCE
TREATMENT RINSE APPLE
anticaries rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61578-315 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 226 ug in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) XYLITOL (UNII: VCQ006KQ1E) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM HYPOCHLORITE (UNII: DY38VHM5OD) Product Characteristics Color white (hazy) Score Shape Size Flavor APPLE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61578-315-01 2 in 1 PACKAGE 05/10/2022 1 175.5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 05/10/2022 TREATMENT RINSE MINT
anticaries rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61578-314 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 226 ug in 1 mL Inactive Ingredients Ingredient Name Strength MENTHOL (UNII: L7T10EIP3A) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) XYLITOL (UNII: VCQ006KQ1E) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM HYPOCHLORITE (UNII: DY38VHM5OD) Product Characteristics Color white (hazy) Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61578-314-01 2 in 1 PACKAGE 05/10/2022 1 175.5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 05/10/2022 Labeler - DENTAL ALLIANCE HOLDINGS LLC (195544965) Registrant - DENTAL ALLIANCE HOLDINGS LLC (195544965) Establishment Name Address ID/FEI Business Operations DENTAL ALLIANCE HOLDINGS LLC 195544965 manufacture(61578-314, 61578-315)