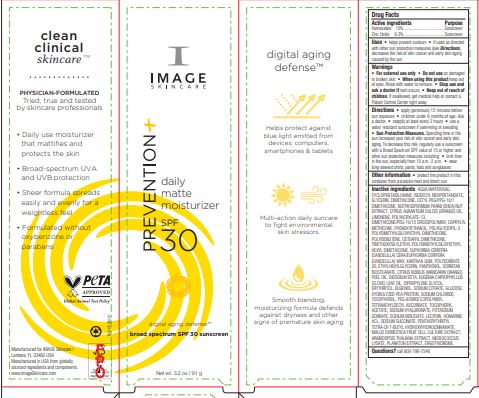
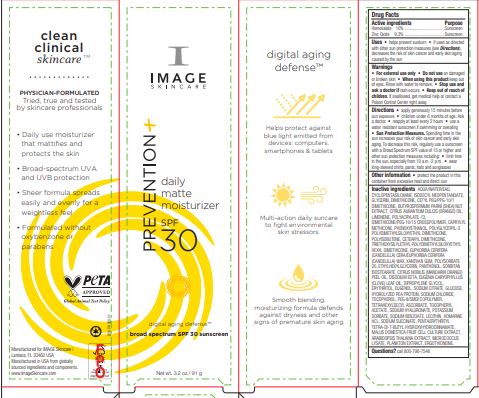
Label: PREVENTION DAILY MATTE MOISTURIZER SPF 30- zinc oxide and homosalate cream
- NDC Code(s): 62742-4223-1, 62742-4223-2, 62742-4223-3
- Packager: Allure Labs
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions • apply generously 15 minutes before sun exposure • children under 6 months of age: Ask a doctor • reapply at least every 2 hours • use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m.-2 p.m. • wear long-sleeved shirts, pants, hats and sunglasses
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients AQUA/WATER/EAU, CYCLOPENTASILOXANE, ISODECYL NEOPENTANOATE, GLYCERIN, DIMETHICONE, CETYL PEG/PPG-10/1 DIMETHICONE, BUTYROSPERMUM PARKII (SHEA) NUT EXTRACT, CITRUS AURANTIUM DULCIS (ORANGE) OIL, LIMONENE, POLYACRYLATE-13, DIMETHICONE/PEG-10/15 CROSSPOLYMER, CAPRYLYL METHICONE, PHENOXYETHANOL, POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE, POLYISOBUTENE, CETEARYL DIMETHICONE, TRIETHOXYSILYLETHYL POLYDIMETHYLSILOXYETHYL HEXYL DIMETHICONE, EUPHORBIA CERIFERA (CANDELILLA) CERA/EUPHORBIA CERIFERA (CANDELILLA) WAX, XANTHAN GUM, POLYSORBATE 20, ETHYLHEXYLGLYCERIN, PANTHENOL, SORBITAN ISOSTEARATE, CITRUS NOBILIS (MANDARIN ORANGE) PEEL OIL, DISODIUM EDTA, EUGENIA CARYOPHYLLUS (CLOVE) LEAF OIL, DIPROPYLENE GLYCOL, ERYTHRITOL, EUGENOL, SODIUM CITRATE, GLUCOSE, HYDROLYZED PEA PROTEIN, SODIUM CHLORIDE, TOCOPHEROL, PEG-8/SMDI COPOLYMER, TETRAHEVLDECYL ASCORBATE, TOCOPHERYL ACETATE, SODIUM HYALURONATE, POTASSIUM SORBATE, SODIUM BENZOATE, LECITHIN, HOMARINE HCL, SODIUM SUCCINATE, PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE, MALUS DOMESTICA FRUIT CELL CULTURE EXTRACT, ARABIDOPSIS THALIANA EXTRACT, MICROCOCCUS LYSATE, PLANKTON EXTRACT, ERGOTHIONEINE.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREVENTION DAILY MATTE MOISTURIZER SPF 30
zinc oxide and homosalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62742-4223 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 9.3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 g Inactive Ingredients Ingredient Name Strength APPLE PEEL (UNII: MA36MNW77O) ARABIDOPSIS THALIANA (UNII: AI3L60HQ81) CLOVE LEAF OIL (UNII: VCA5491KVF) ERGOTHIONEINE (UNII: BDZ3DQM98W) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CITRATE (UNII: 1Q73Q2JULR) AMMONIUM ACRYLOYL DIMETHYLTAURATE/METHACRYLATE, DIMETHYLACRYLAMIDE AND METHACRYLIC ACID COPOLYMER, PPG-3 GLYCERYL TRIACRYLATE CROSSLINKED (100000 MW) (UNII: WR7H9IW2XX) DIMETHICONE/PEG-10/15 CROSSPOLYMER (UNII: 21AS8B1BSS) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CANDELILLA WAX (UNII: WL0328HX19) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) TRIETHOXYSILYLETHYL POLYDIMETHYLSILOXYETHYL HEXYL DIMETHICONE (UNII: X75PL53TZJ) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) EUGENOL (UNII: 3T8H1794QW) SHEANUT (UNII: 84H6HBP32L) POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S) (UNII: RLA2U05Z4Q) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM CHLORIDE (UNII: 451W47IQ8X) PEG-8/SMDI COPOLYMER (UNII: CCX72L6NY6) HOMARINE HYDROCHLORIDE (UNII: 8866LNG61N) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) P-METHYLAMINOPHENOL SULFATE (UNII: D3W0VWG12M) DIMETHICONE (UNII: 92RU3N3Y1O) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 4) (UNII: 8INO2K35FA) POLYISOBUTYLENE (55000 MW) (UNII: TQ77WR8A02) POLYSORBATE 20 (UNII: 7T1F30V5YH) PANTHENOL (UNII: WV9CM0O67Z) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) MANDARIN OIL (UNII: NJO720F72R) DIPROPYLENE GLYCOL (UNII: E107L85C40) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM BENZOATE (UNII: OJ245FE5EU) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ORANGE OIL (UNII: AKN3KSD11B) MICROCOCCUS LUTEUS (UNII: LV6L29Z6AX) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) LIMONENE, (+)- (UNII: GFD7C86Q1W) CETEARYL METHICONE (15000 MW) (UNII: VY9RTR7MSY) ERYTHRITOL (UNII: RA96B954X6) PEA PROTEIN (UNII: 7Q50F46595) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62742-4223-1 7 g in 1 TUBE; Type 0: Not a Combination Product 12/29/2022 2 NDC:62742-4223-3 1 in 1 CARTON 12/29/2022 2 NDC:62742-4223-2 91 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 12/29/2022 Labeler - Allure Labs (926831603) Registrant - Allure Labs (926831603) Establishment Name Address ID/FEI Business Operations Allure Labs 926831603 manufacture(62742-4223)