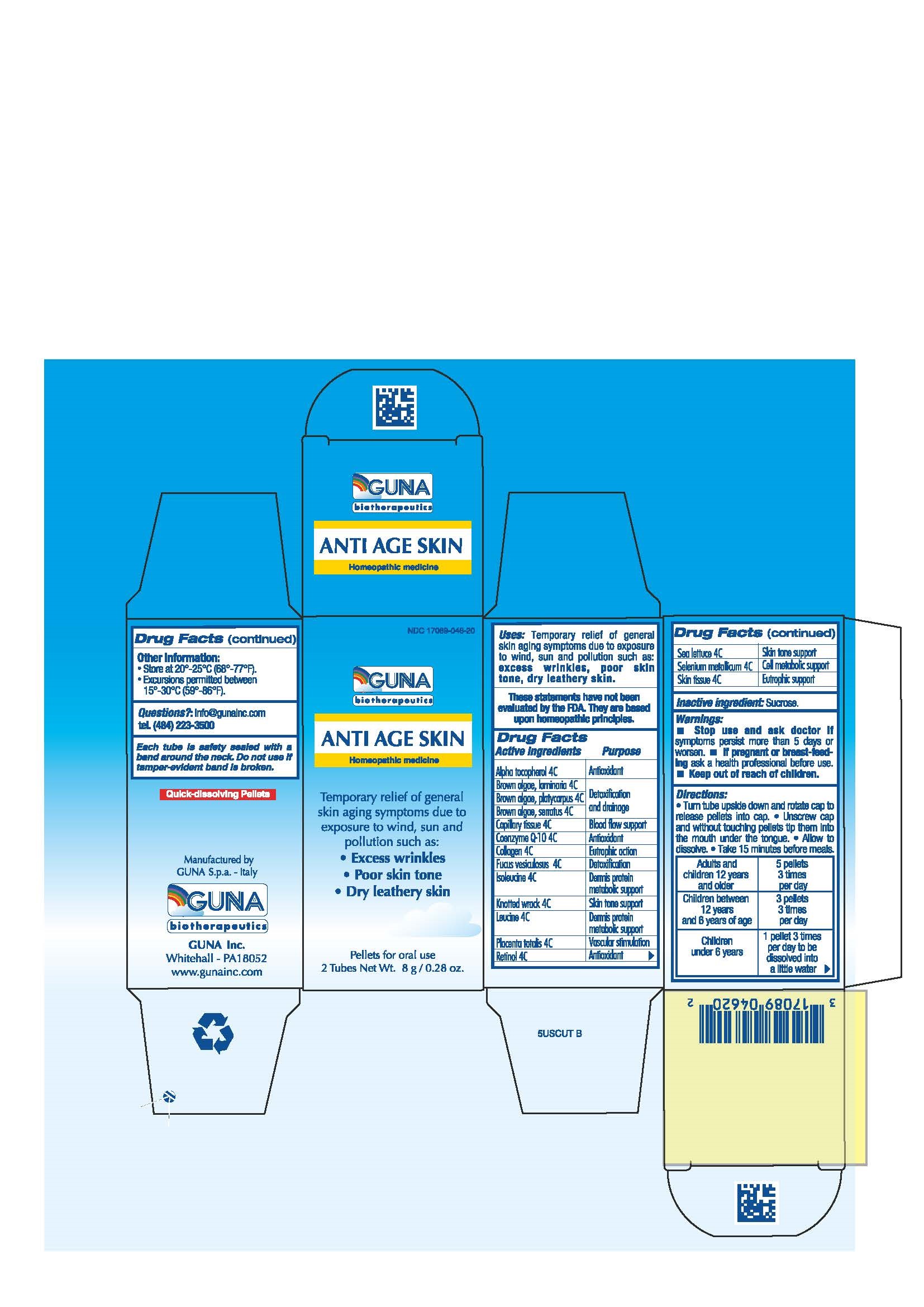
Label: ANTI AGE SKIN- alpha-tocopherol - ascophyllum nodosum - botryoglossum platycarpum - fucus serratus - fucus vesiculosus - isoleucine - leucine - retinol - selenium - sus scrofa capillary tissue - sus scrofa collagen - sus scrofa placenta - sus scrofa skin - ubidecarenone - ulva lactuca extract - laminaria digitata pellet
- NDC Code(s): 17089-046-20
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 21, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS/PURPOSE
ALPHA TOCOPHEROL 4C ANTIOXIDANT
BROWN ALGAE, LAMINARIA 4C DETOXIFICATION AND DRAINAGE
BROWN ALGAE, PLATYCARPUS 4C DETOXIFICATION AND DRAINAGE
BROWN ALGAE, SERRATUS 4C DETOXIFICATION AND DRAINAGE
CAPILLARY TISSUE 4C BLOOD FLOW SUPPORT
COENZYME Q-10 4C ANTIOXIDANT
COLLAGEN 4C EUTROPHIC ACTION
FUCUS VESICULOSUS 4C DETOXIFICATION
ISOLEUCINE 4C DERMIS PROTEIN METABOLIC SUPPORT
KNOTTED WRACK 4C SKIN TONE SUPPORT
LEUCINE 4C DERMIS PROTEIN METABOLIC SUPPORT
PLACENTA TOTALIS 4C VASCULAR STIMULATION
RETINOL 4C ANTIOXIDANT
SEA LETTUCE 4C SKIN TONE
SELENIUM METALLICUM 4C CELL METABOLIC SUPPORT
SKIN TISSUE 4C EUTROPHIC SUPPORT
- USES
- WARNINGS
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTI AGE SKIN
alpha-tocopherol - ascophyllum nodosum - botryoglossum platycarpum - fucus serratus - fucus vesiculosus - isoleucine - leucine - retinol - selenium - sus scrofa capillary tissue - sus scrofa collagen - sus scrofa placenta - sus scrofa skin - ubidecarenone - ulva lactuca extract - laminaria digitata pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-046 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) (ALPHA-TOCOPHEROL - UNII:H4N855PNZ1) ALPHA-TOCOPHEROL 4 [hp_C] in 4 g LAMINARIA DIGITATA (UNII: 15E7C67EE8) (LAMINARIA DIGITATA - UNII:15E7C67EE8) LAMINARIA DIGITATA 4 [hp_C] in 4 g BOTRYOGLOSSUM PLATYCARPUM (UNII: PTA4328M4Z) (BOTRYOGLOSSUM PLATYCARPUM - UNII:PTA4328M4Z) BOTRYOGLOSSUM PLATYCARPUM 4 [hp_C] in 4 g FUCUS SERRATUS (UNII: V8K40WNW5B) (FUCUS SERRATUS - UNII:V8K40WNW5B) FUCUS SERRATUS 4 [hp_C] in 4 g SUS SCROFA CAPILLARY TISSUE (UNII: 253A0356PN) (SUS SCROFA CAPILLARY TISSUE - UNII:253A0356PN) SUS SCROFA CAPILLARY TISSUE 4 [hp_C] in 4 g UBIDECARENONE (UNII: EJ27X76M46) (UBIDECARENONE - UNII:EJ27X76M46) UBIDECARENONE 4 [hp_C] in 4 g SUS SCROFA COLLAGEN (UNII: I8442U2G7J) (SUS SCROFA COLLAGEN - UNII:I8442U2G7J) SUS SCROFA COLLAGEN 4 [hp_C] in 4 g FUCUS VESICULOSUS (UNII: 535G2ABX9M) (FUCUS VESICULOSUS - UNII:535G2ABX9M) FUCUS VESICULOSUS 4 [hp_C] in 4 g ISOLEUCINE (UNII: 04Y7590D77) (ISOLEUCINE - UNII:04Y7590D77) ISOLEUCINE 4 [hp_C] in 4 g ASCOPHYLLUM NODOSUM (UNII: 168S4EO8YJ) (ASCOPHYLLUM NODOSUM - UNII:168S4EO8YJ) ASCOPHYLLUM NODOSUM 4 [hp_C] in 4 g LEUCINE (UNII: GMW67QNF9C) (LEUCINE - UNII:GMW67QNF9C) LEUCINE 4 [hp_C] in 4 g SUS SCROFA PLACENTA (UNII: C8CV8867O8) (SUS SCROFA PLACENTA - UNII:C8CV8867O8) SUS SCROFA PLACENTA 4 [hp_C] in 4 g RETINOL (UNII: G2SH0XKK91) (RETINOL - UNII:G2SH0XKK91) RETINOL 4 [hp_C] in 4 g ULVA LACTUCA EXTRACT (UNII: PHR3P25W6Y) (ULVA LACTUCA EXTRACT - UNII:PHR3P25W6Y) ULVA LACTUCA EXTRACT 4 [hp_C] in 4 g SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 4 [hp_C] in 4 g SUS SCROFA SKIN (UNII: 3EM4VW6TQN) (SUS SCROFA SKIN - UNII:3EM4VW6TQN) SUS SCROFA SKIN 4 [hp_C] in 4 g Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 4 g in 4 g Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-046-20 2 in 1 BOX 12/21/2018 1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-046)