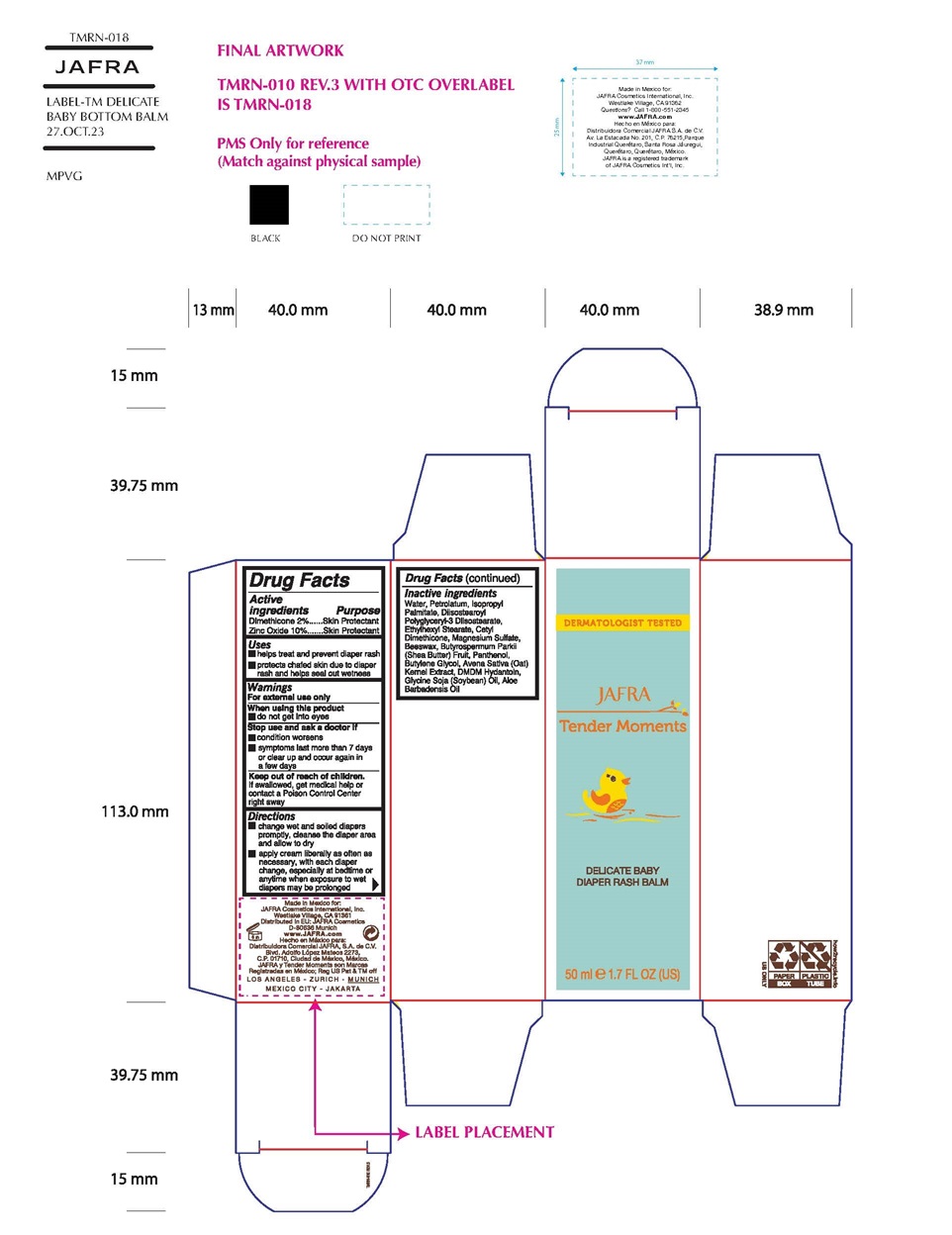
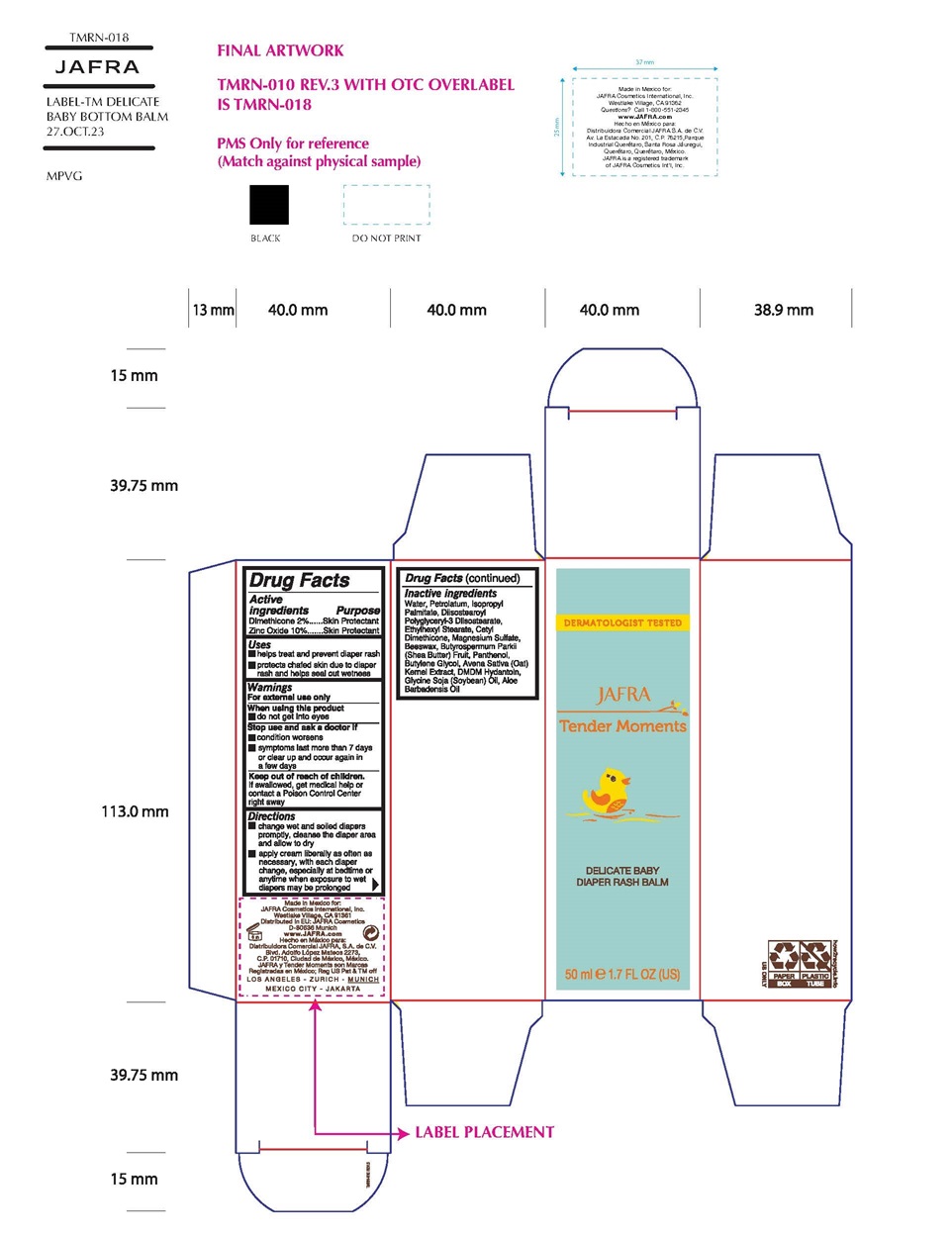
Label: TENDER MOMENTS DELICATE BABY DIAPER RASH BALM- dimethicone,zinc oxide cream
- NDC Code(s): 68828-700-01
- Packager: Jafra Cosmetics International, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive Ingredients
Water/Aqua, Petrolatum, Isopropyl Palmitate, Diisostearoyl Polyglyceryl-3 Dimer Dilinoleate, Ethylhexyl Stearate, Cetyl Dimethicone, Magnesium Sulfate, Beeswax/Cera Alba, Butyrospermum Parkii (Shea Butter), Panthenol, Butylene Glycol, Avena Sativa (Oat) Kernel Extract, DMDM Hydantoin, Glycine Soja (Soybean) Oil, Aloe Barbadensis Oil
- Product label
-
INGREDIENTS AND APPEARANCE
TENDER MOMENTS DELICATE BABY DIAPER RASH BALM
dimethicone,zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-700 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 2 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 10 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) DIISOSTEAROYL POLYGLYCERYL-3 DIMER DILINOLEATE (UNII: G3232Z5S2O) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) WHITE WAX (UNII: 7G1J5DA97F) SHEA BUTTER (UNII: K49155WL9Y) PANTHENOL (UNII: WV9CM0O67Z) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OAT (UNII: Z6J799EAJK) DMDM HYDANTOIN (UNII: BYR0546TOW) SOYBEAN OIL (UNII: 241ATL177A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-700-01 1 in 1 CARTON 12/29/2021 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 12/29/2021 Labeler - Jafra Cosmetics International, Inc. (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-700)