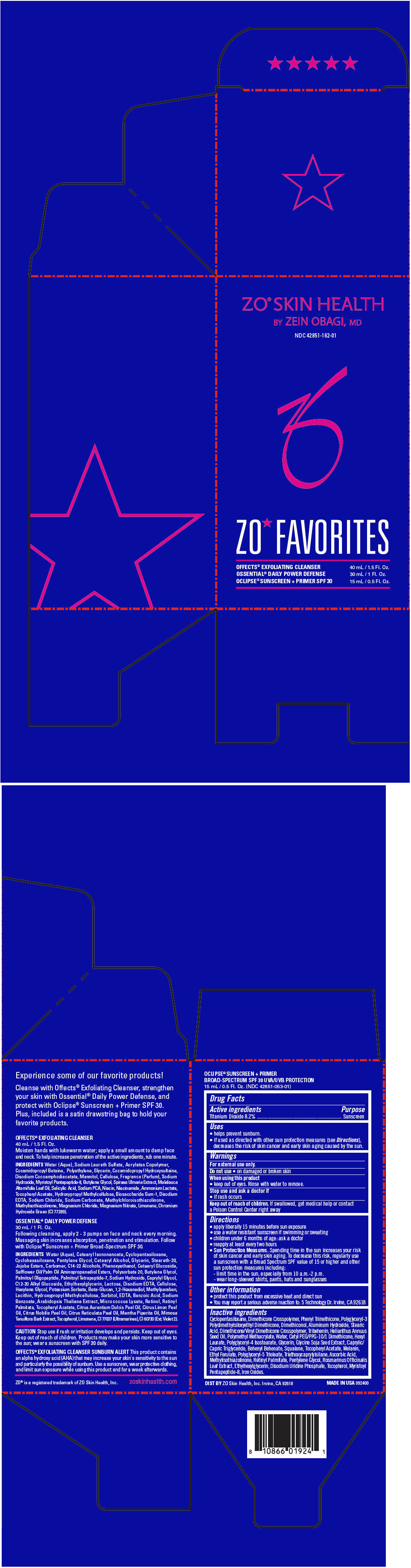
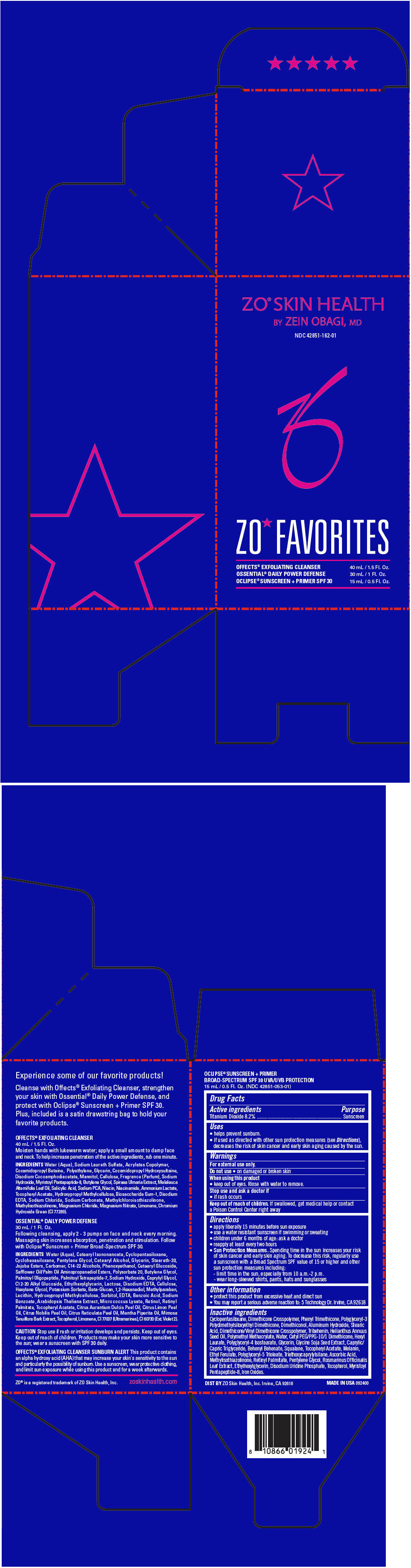
Label: ZO SKIN HEALTH ZO FAVORITES (MOTHERS DAY GWP)- titanium dioxide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 42851-053-01, 42851-162-01 - Packager: ZO Skin Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 16, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
- helps prevent sunburn.
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- children under 6 months of age: ask a doctor
- reapply at least every two hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- Other information
-
Inactive ingredients
Cyclopentasiloxane, Dimethicone Crosspolymer, Phenyl Trimethicone, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, Dimethiconol, Aluminum Hydroxide, Stearic Acid, Dimethicone/Vinyl Dimethicone Crosspolymer, Tribehenin, Helianthus Annuus Seed Oil, Polymethyl Methacrylate, Water, Cetyl PEG/PPG-10/1 Dimethicone, Hexyl Laurate, Polyglyceryl-4 Isostearate, Glycerin, Glycine Soja Seed Extract, Caprylic/Capric Triglyceride, Behenyl Behenate, Squalane, Tocopheryl Acetate, Melanin, Ethyl Ferulate, Polyglyceryl-5 Trioleate, Triethoxycaprylylsilane, Ascorbic Acid, Methylisothiazolinone, Retinyl Palmitate, Pentylene Glycol, Rosmarinus Officinalis Leaf Extract, Ethylhexylglycerin, Disodium Uridine Phosphate, Tocopherol, Myristoyl Pentapeptide-8, Iron Oxides.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
ZO SKIN HEALTH ZO FAVORITES (MOTHERS DAY GWP)
titanium dioxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42851-162 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-162-01 1 in 1 CARTON 04/01/2015 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 40 mL Part 2 1 BOTTLE, PUMP 30 mL Part 3 1 BOTTLE, PUMP 15 mL Part 1 of 3 ZO SKIN HEALTH OFFECTS EXFOLIATING CLEANSER
cleansing (cold creams, cleansing lotions, liquids, and pads) lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR TEA TREE OIL (UNII: VIF565UC2G) INGR WATER (UNII: 059QF0KO0R) INGR SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR COCAMIDOPROPYL HYDROXYSULTAINE (UNII: 62V75NI93W) INGR DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) INGR MANNITOL (UNII: 3OWL53L36A) INGR POWDERED CELLULOSE (UNII: SMD1X3XO9M) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR FILIPENDULA ULMARIA ROOT (UNII: 997724QNDS) INGR SALICYLIC ACID (UNII: O414PZ4LPZ) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR NIACIN (UNII: 2679MF687A) INGR NIACINAMIDE (UNII: 25X51I8RD4) INGR AMMONIUM LACTATE (UNII: 67M901L9NQ) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR HYPROMELLOSES (UNII: 3NXW29V3WO) INGR BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR SODIUM CARBONATE (UNII: 45P3261C7T) INGR METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) INGR METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) INGR MAGNESIUM CHLORIDE (UNII: 02F3473H9O) INGR MAGNESIUM NITRATE (UNII: 77CBG3UN78) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 40 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 04/01/2015 Part 2 of 3 ZO SKIN HEALTH OSSENTIAL DAILY POWER DEFENSE
cleansing (cold creams, cleansing lotions, liquids, and pads) lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR CETEARYL ISONONANOATE (UNII: P5O01U99NI) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR CYCLOMETHICONE 6 (UNII: XHK3U310BA) INGR PENTYLENE GLYCOL (UNII: 50C1307PZG) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR STEARETH-20 (UNII: L0Q8IK9E08) INGR CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) INGR C14-22 ALCOHOLS (UNII: B1K89384RJ) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR POWDERED CELLULOSE (UNII: SMD1X3XO9M) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR 1,2-HEXANEDIOL (UNII: TR046Y3K1G) INGR METHYLPARABEN (UNII: A2I8C7HI9T) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR HYPROMELLOSES (UNII: 3NXW29V3WO) INGR SORBITOL (UNII: 506T60A25R) INGR EDETIC ACID (UNII: 9G34HU7RV0) INGR BENZOIC ACID (UNII: 8SKN0B0MIM) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR ARABIDOPSIS THALIANA (UNII: AI3L60HQ81) INGR RETINOL (UNII: G2SH0XKK91) INGR VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR LEMON OIL (UNII: I9GRO824LL) INGR MANDARIN OIL (UNII: NJO720F72R) INGR PEPPERMINT OIL (UNII: AV092KU4JH) INGR MIMOSA TENUIFLORA BARK (UNII: 515MQE449I) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR ULTRAMARINE BLUE (UNII: I39WR998BI) INGR EXT. D&C VIOLET NO. 2 (UNII: G5UX3K0728) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 04/01/2015 Part 3 of 3 ZO SKIN HEALTH OCLIPSE SUNSCREEN PLUS PRIMER BROAD-SPECTRUM SPF 30 UVA/UVB PROTECTION
titanium dioxide lotionProduct Information Item Code (Source) NDC:42851-053 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 82 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S) (UNII: RLA2U05Z4Q) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIBEHENIN (UNII: 8OC9U7TQZ0) SUNFLOWER OIL (UNII: 3W1JG795YI) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) WATER (UNII: 059QF0KO0R) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) GLYCERIN (UNII: PDC6A3C0OX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) BEHENYL BEHENATE (UNII: K8NU647RJ0) SQUALANE (UNII: GW89575KF9) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MELANIN SYNTHETIC (TYROSINE, PEROXIDE) (UNII: O0CV1RMR44) ETHYL FERULATE (UNII: 5B8915UELW) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ASCORBIC ACID (UNII: PQ6CK8PD0R) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PENTYLENE GLYCOL (UNII: 50C1307PZG) ROSEMARY (UNII: IJ67X351P9) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) URIDINE MONOPHOSPHATE DISODIUM (UNII: KD8E20071T) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-053-01 15 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/01/2015 Labeler - ZO Skin Health, Inc. (826468527) Establishment Name Address ID/FEI Business Operations Lifetech Resources, LLC. 081239587 manufacture(42851-162) Establishment Name Address ID/FEI Business Operations Thibiant International, Inc. 083913913 manufacture(42851-162) Establishment Name Address ID/FEI Business Operations PakLab 790530976 manufacture(42851-162)