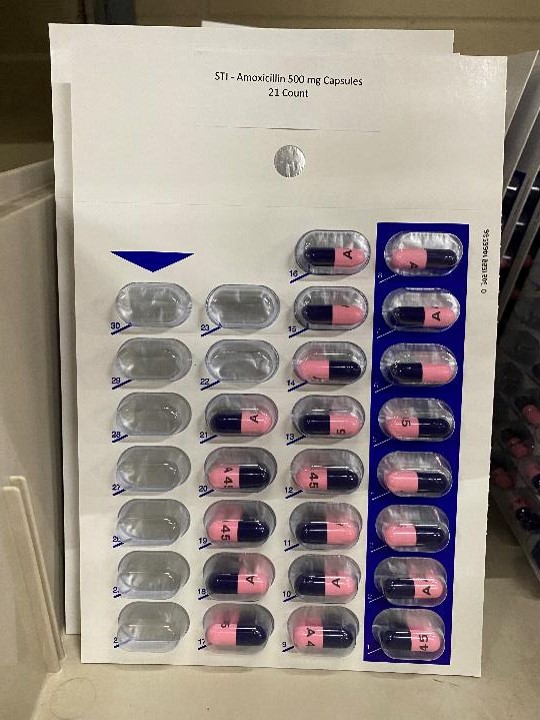
Label: AMOXICILLIN 500 MG- amoxicillin capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 83112-031-21, 83112-031-30 - Packager: Health Department, Oklahoma State
- This is a repackaged label.
- Source NDC Code(s): 57237-031
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Precaustions from Manufacturer Package Insert
5.1 Anaphylactic Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy including amoxicillin. Although anaphylaxis is more frequent following parenteral therapy, it has occurred in patients on oral penicillins. These reactions are more likely to occur in individuals with a history of penicillin hypersensitivity and/or a history of sensitivity to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins. Before initiating therapy with amoxicillin, careful inquiry should be made regarding previous hypersensitivity reactions to penicillins, cephalosporins, or other allergens. If an allergic reaction occurs, amoxicillin should be discontinued and appropriate therapy instituted.
5.2 Clostridium difficile Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including amoxicillin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.5.3 Development of Drug-Resistant Bacteria
Prescribing amoxicillin in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.4 Use in Patients With Mononucleosis
A high percentage of patients with mononucleosis who receive amoxicillin develop an erythematous skin rash. Thus amoxicillin should not be administered to patients with mononucleosis.
- Repackaging Label
-
INGREDIENTS AND APPEARANCE
AMOXICILLIN 500 MG
amoxicillin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:83112-031(NDC:57237-031) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMOXICILLIN (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 500 mg Product Characteristics Color blue, pink (Pink/blue capsules) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code A45 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83112-031-21 21 in 1 BLISTER PACK; Type 0: Not a Combination Product 01/01/2023 2 NDC:83112-031-30 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 01/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065271 01/01/2023 Labeler - Health Department, Oklahoma State (143673015)