Label: BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 DEEP MAHOGANY- titanium dioxide liquid
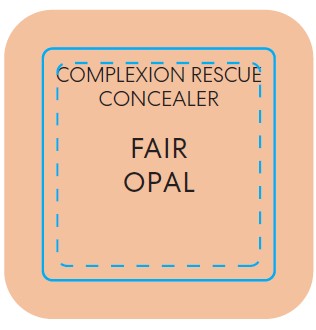
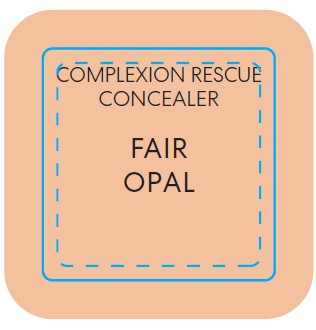
BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 FAIR OPAL- titanium dioxide liquid
BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 FAIR BIRCH- titanium dioxide liquid
BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 .......NING CONCEALER SPF 25 DEEP SIENNA- titanium dioxide liquid
BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 DEEP CINNAMON- titanium dioxide liquid
BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 FAIR VANILLA- titanium dioxide liquid
BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 LIGHT CASHEW- titanium dioxide liquid
-
NDC Code(s):
98132-284-01,
98132-285-01,
98132-286-01,
98132-287-01, view more98132-288-01, 98132-289-01, 98132-290-01, 98132-291-01, 98132-292-01, 98132-293-01, 98132-294-01, 98132-295-01, 98132-296-01, 98132-297-01, 98132-298-01
- Packager: Orveon Global US LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- Active Ingredient Purpose Titanium Dioxide 6.15% Sunscreen
- Uses
- Warnings
- Do not use • on damaged or broken skin.
- When using this product • keep out of eyes. Rince with water to remove.
- Stop use and ask a doctor if • rash occurs.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Directions
-
Inactive ingredients
WATER, COCONUT ALKANES, PROPANEDIOL, SQUALANE, SORBITAN SESQUIISOSTEARATE, ISOSTEARIC ACID, POLYSORBATE 60, TREHALOSE, GLYCERIN, AGAR, SILICA, MORINGA OLEIFERA SEED EXTRACT, SALICORNIA HERBACEA EXTRACT, MELILOTUS OFFICINALIS EXTRACT, THEOBROMA CACAO (COCOA) SEED EXTRACT, COCO-CAPRYLATE /CAPRATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, CAFFEINE, LAUROYL LYSINE, SUCCINOGLYCAN, ALUMINUM
HYDROXIDE, HYDROGEN DIMETHICONE, CITRIC ACID, DISODIUM PHOSPHATE, ACETYL TETRAPEPTIDE-5, BUTYLENE
GLYCOL, POLYGLYCERYL-4 LAURATE/SUCCINATE, CELLULOSE GUM, MAGNESIUM CHLORIDE, CALCIUM CHLORIDE, POTASSIUM CHLORIDE, SODIUM HYALURONATE, MAGNESIUM STEARATE, PHENOXYETHANOL, MICA, IRON OXIDES, TITANIUM DIOXIDE.
- Other information
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
- Principal Display Panel - 10 mL / 0.338 fl. oz. liq. US
-
INGREDIENTS AND APPEARANCE
BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 DEEP MAHOGANY
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-284 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) TREHALOSE (UNII: B8WCK70T7I) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-284-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 FAIR OPAL
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-285 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-285-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 FAIR BIRCH
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-287 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-287-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 LIGHT BAMBOO
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-289 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-289-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 MEDIUM WHEAT
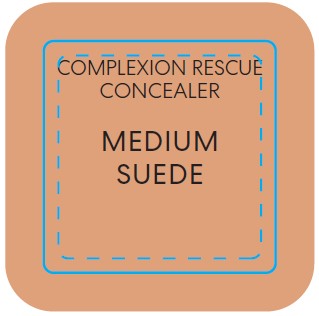
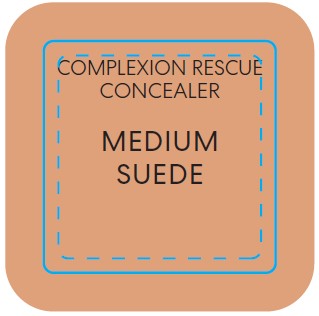
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-290 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-290-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 MEDIUM SUEDE
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-291 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-291-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 MEDIUM NATURAL PECAN
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-292 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-292-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 MEDIUM DEEP TAN AMBER
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-293 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-293-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 MEDIUM DEEP DESERT
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-294 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-294-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 MEDIUM DEEP SPICE
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-295 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-295-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 DEEP CHESTNUT
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-296 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-296-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 DEEP SIENNA
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-297 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-297-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 DEEP CINNAMON
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-298 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-298-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 FAIR VANILLA
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-286 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-286-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 BAREMINERALS COMPLEXION RESCUE BRIGHTENING CONCEALER SPF 25 LIGHT CASHEW
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-288 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.065 g in 10 mL Inactive Ingredients Ingredient Name Strength CAFFEINE (UNII: 3G6A5W338E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) MELILOTUS OFFICINALIS WHOLE (UNII: DJR90OLD7P) COCOA (UNII: D9108TZ9KG) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) AGAR (UNII: 89T13OHQ2B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TREHALOSE (UNII: B8WCK70T7I) PROPANEDIOL (UNII: 5965N8W85T) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MORINGA OLEIFERA SEED (UNII: TIX5482832) SALICORNIA EUROPAEA WHOLE (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LAUROYL LYSINE (UNII: 113171Q70B) ACETYL TETRAPEPTIDE-5 (UNII: Y1DFQ308G8) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM STEARATE (UNII: 70097M6I30) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERROUS OXIDE (UNII: G7036X8B5H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-288-01 1 in 1 CARTON 12/02/2022 1 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/02/2022 Labeler - Orveon Global US LLC (118344494)