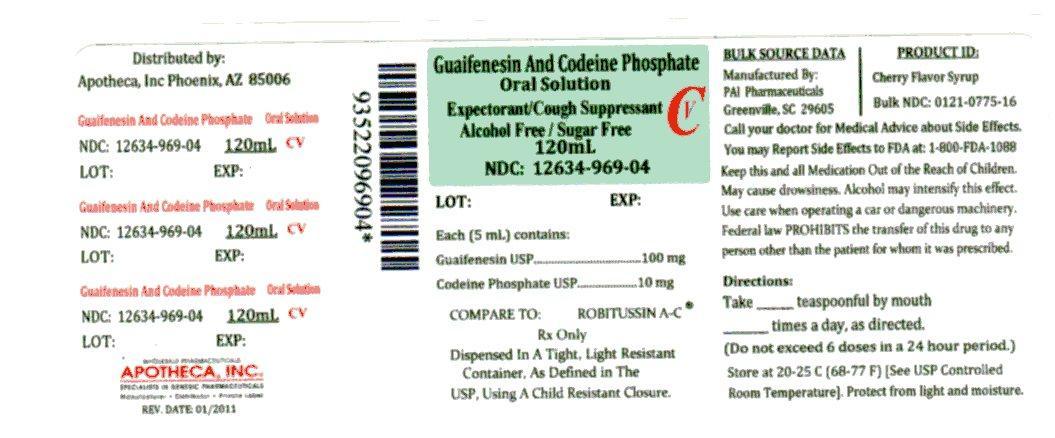
Label: GUAIFENESIN AND CODEINE PHOSPHATE solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 12634-969-04 - Packager: Apotheca, Inc
- This is a repackaged label.
- Source NDC Code(s): 0121-1775
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: CV
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 3, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
ABOUT
ACTIONS
This product combines the expectorant, guaifenesin, with the cough suppressant, codeine. Guaifenesin enhances the output of lower respiratory tract fluid. The enhanced flow of less viscid secretions promotes and facilitates the removal of mucus. Codeine is a centrally acting agent which elevates the threshold for cough.
As a result, dry, unproductive coughs become more productive and less frequent.
INDICATIONS
Temporarily controls cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants. Helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive.
CONTRAINDICATIONS
Hypersensitivity to any of the ingredients.
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
A persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash or persistent headache, consult a physician. Do not take this product for persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, emphysema, or if cough is accompanied by excessive phlegm (mucus) unless directed by a physician. Adults and children who have a chronic pulmonary disease or shortness of breath, or children who are taking other drugs, should not take this product unless directed by a physician. May cause or aggravate constipation. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Professional Note: Guaifenesin has been shown to produce a color interference with certain clinical laboratory determinations of 5-hydroxyindoleacetic acid (5-HIAA) and vanillylmandelic acid (VMA).
DRUG INTERACTION PRECAUTION
Caution should be used when taking this product with sedatives, tranquilizers and drugs used for depression, especially monoamine oxidase inhibitors (MAOIs). These combinations may cause greater sedation (drowsiness) than is caused by the products used alone. (See WARNINGS)
-
DOSAGE & HOW SUPPLIED
Take orally as stated below or use as directed by a physician.
Adults and children 12 years of age and over:10 mL (2 teaspoonfuls) every 4 hours, not to exceed 12 teaspoonfuls in a 24-hour period; Children 6 to under 12 years:5 mL (1 teaspoonful) every 4 hours, not to exceed 6 teaspoonfuls in a 24-hour period;
Children under 6 years: Consult a physician. A special measuring device should be used to give an accurate dose of this product to children under 6 years of age.
Giving a higher dose than recommended by a physician could result in serious side effects for a child.
Use of codeine-containing preparations is not recommended for children under 2 years of age. Do not exceed recommended dosage.
STORAGE
Keep tightly closed. Store at controlled room temperature, 20°-25°C (68°-77°F). Protect from light.
HOW SUPPLIED
Guaifenesin and Codeine Phosphate Oral Solution USP (red color-cherry flavor) is supplied in the following oral dosage forms:
NDC 12634-969-04 (4 fl oz (120mL) bottle
Manufactured by:
Pharmaceutical Associates, Inc.
Greenville, SC 29605Distrubited by:
Apotheca, Inc.
Phoenix, AZ 85006
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN AND CODEINE PHOSPHATE
guaifenesin and codeine phosphate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:12634-969(NDC:0121-1775) Route of Administration oral DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Guaifenesin (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) Guaifenesin 100 mg in 5 mL CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) CODEINE PHOSPHATE 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength MENTHOL (UNII: L7T10EIP3A) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12634-969-04 120 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/28/2011 Labeler - Apotheca, Inc (051457844) Establishment Name Address ID/FEI Business Operations Apotheca, Inc 051457844 relabel(12634-969)