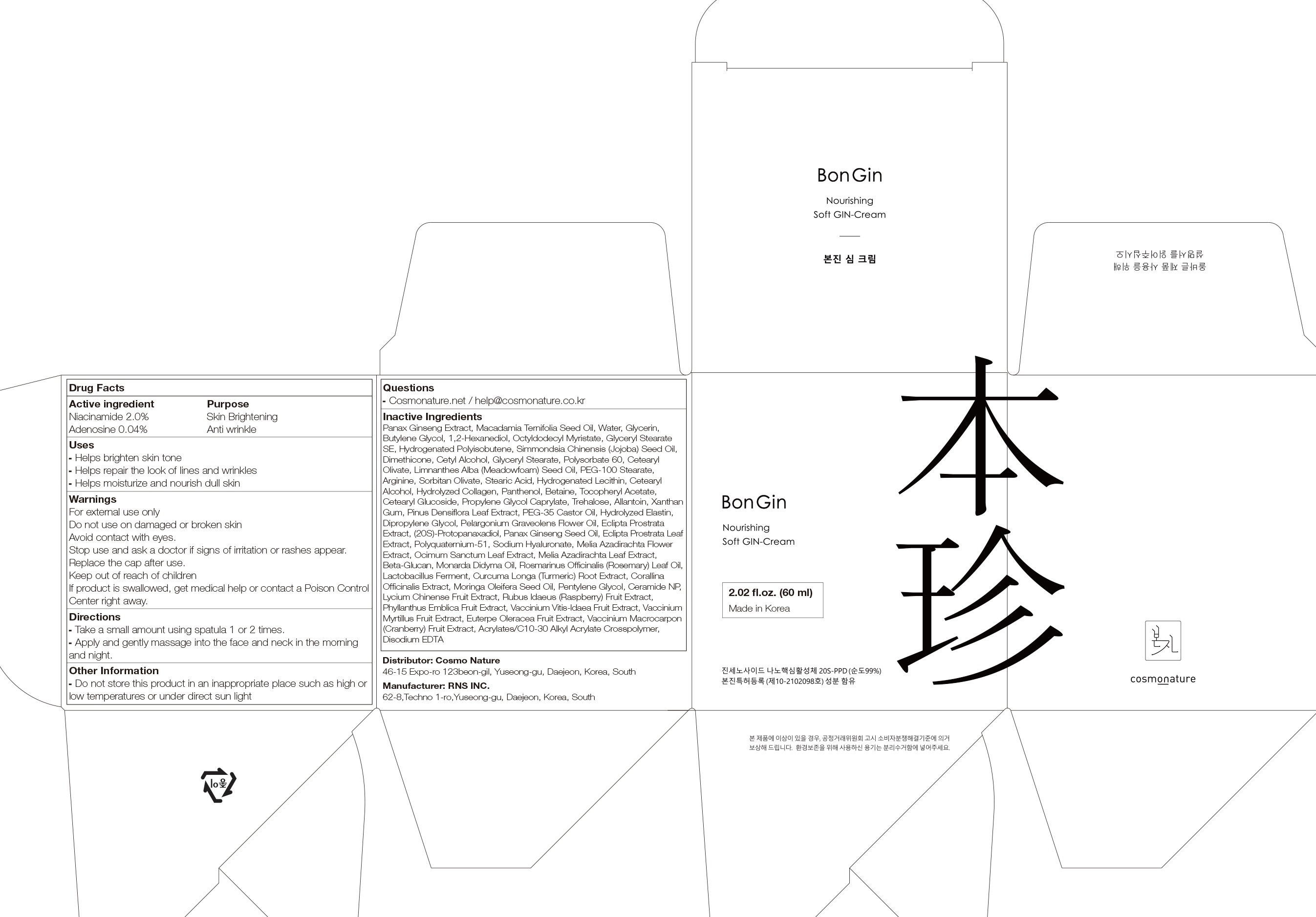
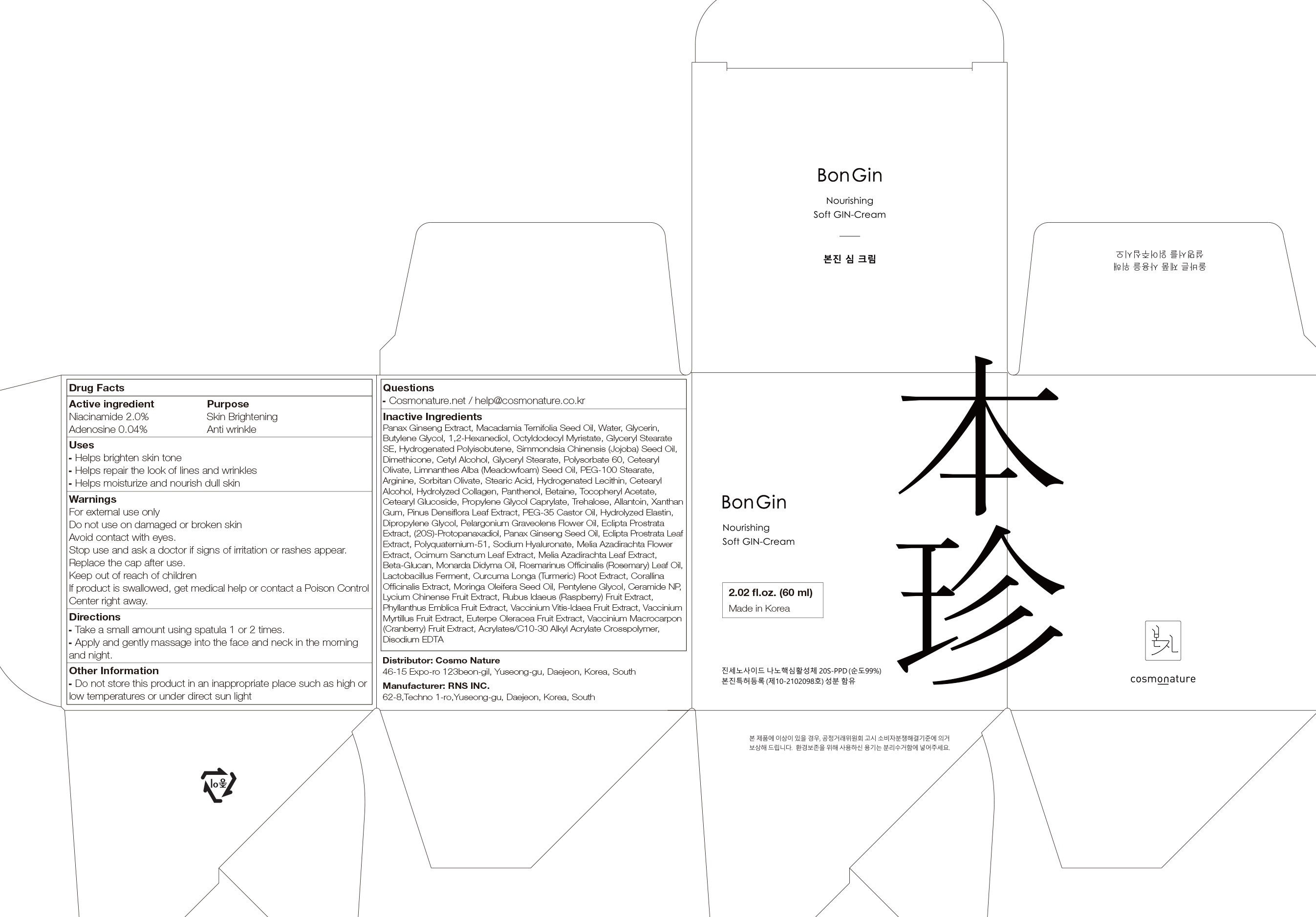
Label: BONGIN REBALANCING GIN- niacinamide, adenosine cream
- NDC Code(s): 82492-030-01, 82492-030-02
- Packager: Cosmo Nature
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
-
INACTIVE INGREDIENTS
Panax Ginseng Extract, Macadamia Ternifolia Seed Oil, Water, Glycerin, Butylene Glycol, 1,2-Hexanediol, Octyldodecyl Myristate, Glyceryl Stearate SE, Hydrogenated Polyisobutene, Simmondsia Chinensis (Jojoba) Seed Oil, Dimethicone, Cetyl Alcohol, Glyceryl Stearate, Polysorbate 60, Cetearyl Olivate, Limnanthes Alba (Meadowfoam) Seed Oil, PEG-100 Stearate, Arginine, Sorbitan Olivate, Stearic Acid, Hydrogenated Lecithin, Cetearyl Alcohol, Hydrolyzed Collagen, Panthenol, Betaine, Tocopheryl Acetate, Cetearyl Glucoside, Propylene Glycol Caprylate, Trehalose, Allantoin, Xanthan Gum, Pinus Densiflora Leaf Extract, PEG-35 Castor Oil, Hydrolyzed Elastin, Dipropylene Glycol, Pelargonium Graveolens Flower Oil, Eclipta Prostrata Extract, (20S)-Protopanaxadiol, Panax Ginseng Seed Oil, Eclipta Prostrata Leaf Extract, Polyquaternium-51, Sodium Hyaluronate, Melia Azadirachta Flower Extract, Ocimum Sanctum Leaf Extract, Melia Azadirachta Leaf Extract, Beta-Glucan, Monarda Didyma Oil, Rosmarinus Officinalis (Rosemary) Leaf Oil, Lactobacillus Ferment, Curcuma Longa (Turmeric) Root Extract, Corallina Officinalis Extract, Moringa Oleifera Seed Oil, Pentylene Glycol, Ceramide NP, Lycium Chinense Fruit Extract, Rubus Idaeus (Raspberry) Fruit Extract, Phyllanthus Emblica Fruit Extract, Vaccinium Vitis-Idaea Fruit Extract, Vaccinium Myrtillus Fruit Extract, Euterpe Oleracea Fruit Extract, Vaccinium Macrocarpon (Cranberry) Fruit Extract, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Disodium EDTA
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- Other Information
- Questions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BONGIN REBALANCING GIN
niacinamide, adenosine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82492-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) Niacinamide 2.0 g in 100 mL Adenosine (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) Adenosine 0.04 g in 100 mL Inactive Ingredients Ingredient Name Strength MACADAMIA OIL (UNII: 515610SU8C) Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) 1,2-Hexanediol (UNII: TR046Y3K1G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82492-030-02 1 in 1 CARTON 02/01/2022 1 NDC:82492-030-01 60 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2022 Labeler - Cosmo Nature (688982617) Registrant - Cosmo Nature (688982617) Establishment Name Address ID/FEI Business Operations BIOCM CO.,LTD 688478040 manufacture(82492-030)