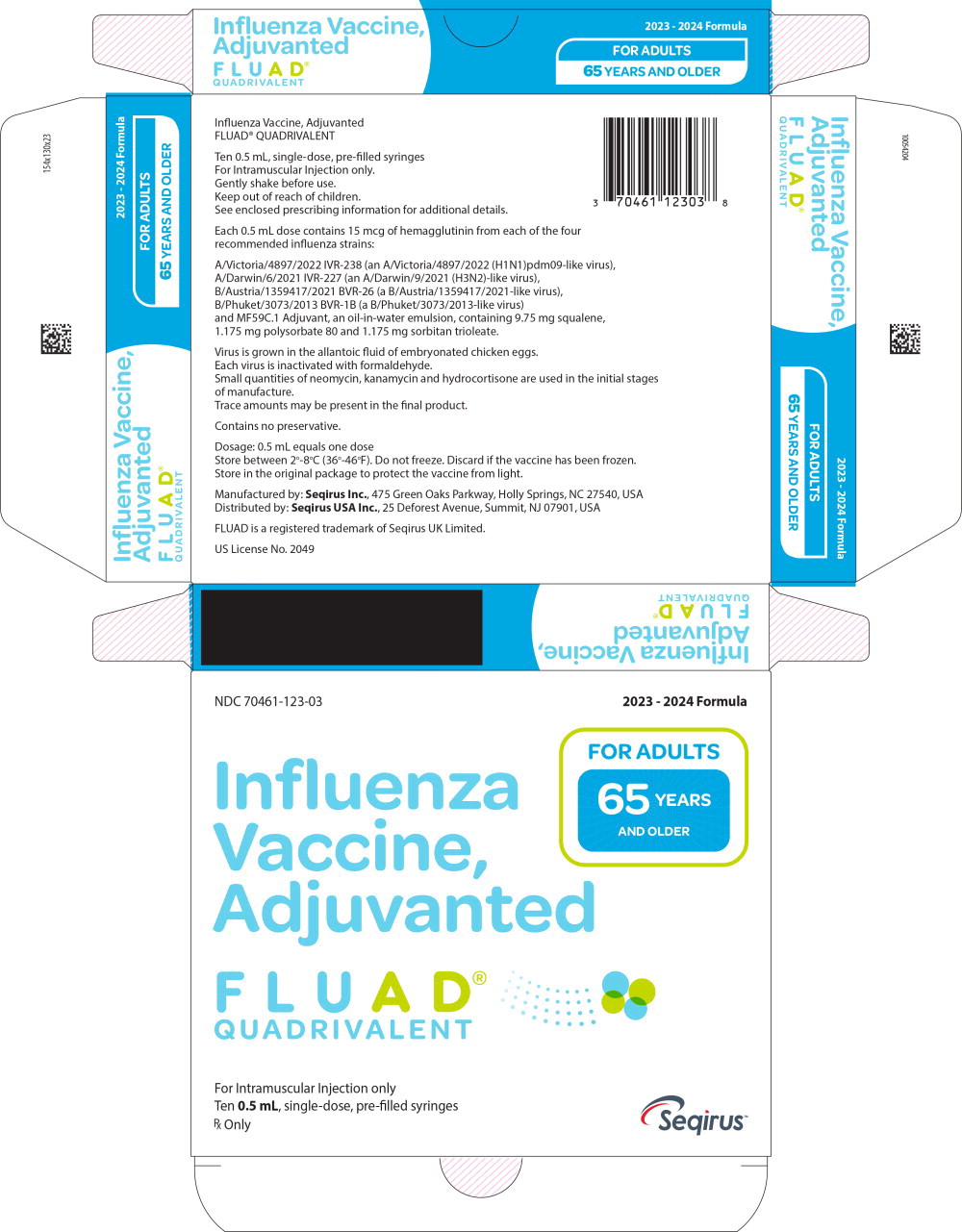
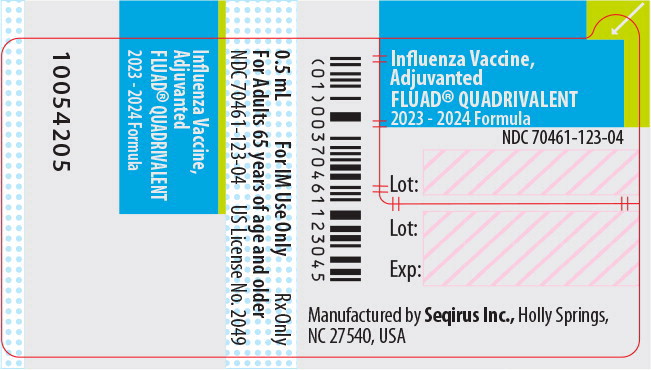
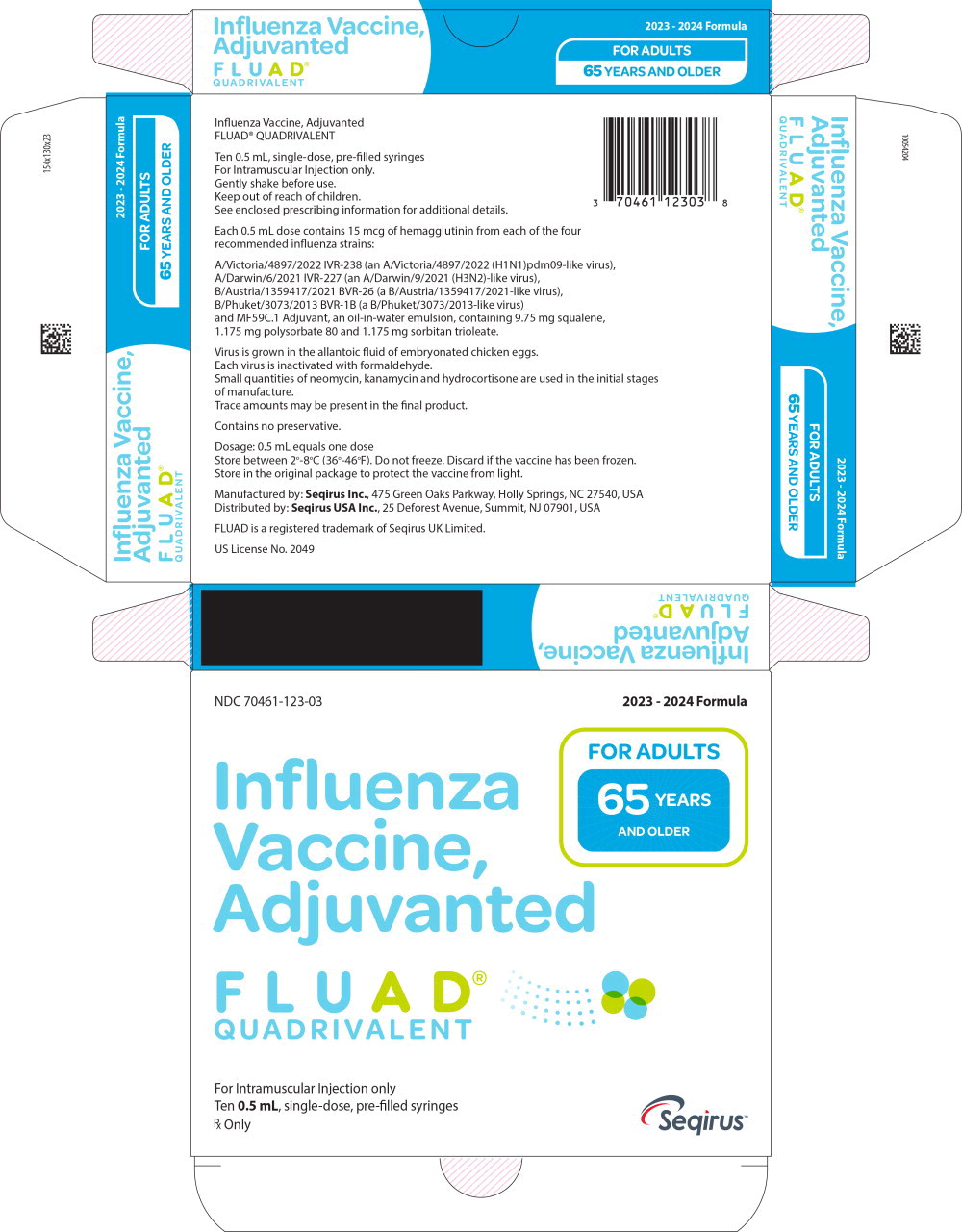
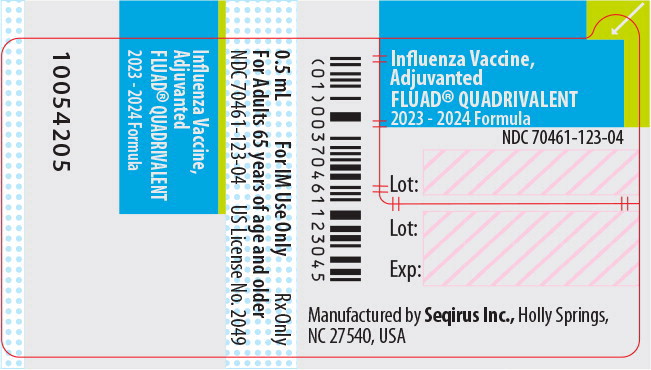
Label: FLUAD QUADRIVALENT (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/darwin/6/2021 ivr-227 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/austria/1359417/2021 bvr-26 antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 bvr-1b antigen- formaldehyde inactivated injection, suspension
- NDC Code(s): 70461-123-03, 70461-123-04
- Packager: Seqirus, Inc.
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FLUAD® QUADRIVALENT safely and effectively. See full prescribing information for FLUAD QUADRIVALENT.
FLUAD QUADRIVALENT (Influenza Vaccine, Adjuvanted)
Injectable Emulsion for Intramuscular Use
2023-2024 Formula
Initial U.S. Approval: 2020INDICATIONS AND USAGE
FLUAD QUADRIVALENT is an inactivated influenza vaccine indicated for active immunization against influenza disease caused by influenza virus subtypes A and types B contained in the vaccine. FLUAD QUADRIVALENT is approved for use in persons 65 years of age and older. (1)
This indication is approved under accelerated approval based on the immune response elicited by FLUAD QUADRIVALENT (1). Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
DOSAGE AND ADMINISTRATION
A single 0.5 mL dose for intramuscular injection. (2.1)
DOSAGE FORMS AND STRENGTHS
Injectable emulsion supplied in 0.5 mL single-dose pre-filled syringes. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
If Guillain-Barré Syndrome (GBS) has occurred within six weeks of previous influenza vaccination, the decision to give FLUAD QUADRIVALENT should be based on careful consideration of the potential benefits and risks. (5.1)
ADVERSE REACTIONS
The most common (≥ 10%) local and systemic reactions in elderly subjects 65 years of age and older were injection site pain (16.3%), headache (10.8%) and fatigue (10.5%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Seqirus at 1-855-358-8966 or VAERS at 1-800-822-7967 and www.vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Schedule
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
5.2 Preventing and Managing Allergic Reactions
5.3 Altered Immunocompetence
5.4 Syncope
5.5 Limitations of Vaccine Effectiveness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Concomitant Use With Other Vaccines
7.2 Concurrent Use With Immunosuppressive Therapies
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Immunogenicity of FLUAD QUADRIVALENT
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
FLUAD QUADRIVALENT is an inactivated influenza vaccine indicated for active immunization against influenza disease caused by influenza virus subtypes A and types B contained in the vaccine. FLUAD QUADRIVALENT is approved for use in persons 65 years of age and older. This indication is approved under accelerated approval based on the immune response elicited by FLUAD QUADRIVALENT [see Clinical Studies (14.1)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
-
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only
2.1 Dosage and Schedule
Administer FLUAD QUADRIVALENT as a single 0.5 mL intramuscular injection in adults 65 years of age and older.
2.2 Administration
- Gently shake each syringe. FLUAD QUADRIVALENT has a milky-white appearance. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit [see Description (11)]. If either condition exists, FLUAD QUADRIVALENT should not be administered.
- To use a pre-filled syringe fitted with a Luer Lok system, remove the tip cap by unscrewing it in a counter-clockwise direction. Once the tip cap is removed, attach a needle to the syringe by screwing it on in a clockwise direction until it locks. Once the needle is locked in place, remove the needle protector and administer the vaccine.
- The vaccine should be administered by intramuscular injection, preferably in the region of the deltoid muscle of the upper arm. Do not inject the vaccine in the gluteal region or areas where there may be a major nerve trunk.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer FLUAD QUADRIVALENT to anyone with a history of severe allergic reaction (e.g. anaphylaxis) to any component of the vaccine, including egg protein [see Description (11)], or to a previous influenza vaccine.
-
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
If Guillain-Barré syndrome (GBS) has occurred within 6 weeks of receipt of prior influenza vaccine, the decision to give FLUAD QUADRIVALENT should be based on careful consideration of the potential benefits and risks. The 1976 swine influenza vaccine was associated with an elevated risk of GBS. [see References (1)] Evidence for a causal relationship of GBS with other influenza vaccines is inconclusive; if an excess risk exists, it is probably slightly more than 1 additional case per 1 million persons vaccinated.
5.2 Preventing and Managing Allergic Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
5.3 Altered Immunocompetence
The immune response to FLUAD QUADRIVALENT in immunocompromised persons, including individuals receiving immunosuppressive therapy, may be lower than in immunocompetent individuals. [see Concurrent Use With Immunosuppressive Therapies (7.2)]
-
6 ADVERSE REACTIONS
The most common (≥10%) local and systemic reactions in elderly subjects 65 years of age and older were injection site pain (16.3%), headache (10.8%) and fatigue (10.5%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect rates observed in clinical practice.
The safety of FLUAD QUADRIVALENT was evaluated in two clinical studies in 4269 elderly subjects 65 years of age and older. Study 1 (NCT02587221) was a multi-center, randomized, observer-blind, non-influenza comparator-controlled efficacy and safety study conducted in 12 countries during the 2016-2017 Northern Hemisphere and 2017 Southern Hemisphere seasons. In this study, 3381 subjects received FLUAD QUADRIVALENT and 3380 subjects received a US-licensed non-influenza comparator vaccine (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Boostrix® [GlaxoSmithKline Biologicals]).
The mean age of subjects at enrollment was 72 years, 62% were female, 48% White, 34% Asian, 16% Other, 2% American Indian/Alaska Native, and 18% of Hispanic/Latino ethnicity.
Solicited local and systemic adverse reactions were collected for 7 days after vaccination in a subset of 665 subjects who received FLUAD QUADRIVALENT and 667 subjects who received the comparator vaccine. The percentages of subjects reporting solicited local adverse reactions are presented in Table 1a and systemic adverse reactions are presented in Table 1b. Onset usually occurred within the first 2 days after vaccination. The majority of solicited reactions resolved within 3 days.
Table 1a. Percentages of Subjects Reporting Solicited Local Adverse Reactionsa in the Solicited Safety Populationb within 7 Days of Vaccination (Study 1) Study 1: NCT02587221
Abbreviation: N=number of subjects with solicited safety data
Non-Influenza Comparator Vaccine = combined Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Boostrix® (GlaxoSmithKline Biologicals)
a All solicited local adverse events reported within 7 days of vaccination are included
b Solicited Safety Population: all subjects in the exposed population who received a study vaccine and provided post-vaccination solicited safety data
c Severe reactions of each type were reported in 1.1% or fewer subjects receiving FLUAD QUADRIVALENT; severe reactions of each type were also reported in the comparator group at similar percentages. Severe definitions: Erythema, Induration and Ecchymosis = >100 mm diameter; Injection site pain, = prevents daily activity.
Local (Injection site) Reactionsc FLUAD QUADRIVALENT
N=595-659Non-Influenza Comparator Vaccine
N=607-664Injection site pain 16.3 11.2 Erythema ≥25mm 3.8 1.8 Induration ≥25mm 4.0 2.6 Ecchymosis ≥25mm 0.5 0.7 Table 1b. Percentages of Subjects Reporting Solicited Systemic Adverse Reactionsa in the Solicited Safety Populationb within 7 Days of Vaccination (Study 1) Systemic Reactionsc FLUAD QUADRIVALENT
N=595-659Non-Influenza Comparator Vaccine
N=607-664Study 1: NCT02587221
Abbreviation: N=number of subjects with solicited safety data
Non-Influenza Comparator Vaccine = combined Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Boostrix® (GlaxoSmithKline Biologicals)
a All solicited systemic adverse events reported within 7 days of vaccination are included
b Solicited Safety Population: all subjects in the exposed population who received a study vaccine and provided post-vaccination solicited safety data
c Severe reactions of each type were reported in 1.1% or fewer subjects receiving FLUAD QUADRIVALENT; severe reactions of each type were also reported in the comparator group at similar percentages. Severe definitions: Nausea, Fatigue, Myalgia, Arthralgia, Headache, and Chills = prevents daily activity; Loss of appetite = not eating at all; Vomiting = 6 or more times in 24 hours or requires intravenous hydration; Diarrhea = 6 or more loose stools in 24 hours or requires intravenous hydration; Fever = ≥102.2°F (39°C).
Headache 10.8 8.3 Fatigue 10.5 8.8 Myalgia 7.7 6.1 Arthralgia 7.3 6.6 Chills 5.0 3.9 Diarrhea 4.1 3.0 Nausea 3.8 2.3 Loss of appetite 3.6 3.6 Fever ≥100.4°F (38°C) 1.7 1.2 Vomiting 0.8 1.1 Unsolicited adverse events (AEs) were collected for all subjects for 21 days after vaccination. Related unsolicited AEs were reported by 303 (9.0%) and by 261 (7.7%) of the subjects for FLUAD QUADRIVALENT and Boostrix, respectively. For FLUAD QUADRIVALENT, injection site pain and influenza-like illness were the only unsolicited adverse reactions reported in ≥ 1% of subjects (1.7% and 1.5%, respectively).
Serious adverse events (SAEs) and potentially immune-mediated adverse events of special interest (AESIs) were collected up to 366 days after vaccination. SAEs were reported by 238 (7.0%) FLUAD QUADRIVALENT recipients and 234 (6.9%) comparator recipients. There were no SAEs, AESIs or deaths in this study that were related to FLUAD QUADRIVALENT.
Study 2 (NCT03314662) was a multicenter, randomized, double-blind, comparator-controlled study conducted during the 2017-18 Northern Hemisphere influenza season. In this study, 888 subjects received FLUAD QUADRIVALENT, 444 subjects received the licensed adjuvanted trivalent vaccine (aTIV-1 - FLUAD® (trivalent formulation)) and 444 subjects received an adjuvanted trivalent influenza vaccine with an alternate B strain (aTIV-2).
The mean age of subjects at enrollment who received FLUAD QUADRIVALENT was 72.5 years. Female subjects represented 56.6% of the study population and the racial distribution of subjects was 91.6% Caucasian, 7.0% Black or African American, and ≤ 1% each for Asian, Native Hawaiian or Pacific Islander, American Indian or Alaska Native or Other.
Solicited local and systemic adverse reactions reported within 7 days after vaccination were similar to those reported for Study 1. Unsolicited AEs were collected for 21 days after vaccination. Related unsolicited AEs were reported by 39 (4.4%) and by 17-19 (3.8%-4.3%) of subjects administered FLUAD QUADRIVALENT or aTIV, respectively. For FLUAD QUADRIVALENT, injection site bruising (1.0%) was the only unsolicited adverse reaction reported in ≥ 1% of subjects.
Serious AEs and AESIs were collected up to 181 days after vaccination. Within 6 months after vaccination, 37 (4.2%) FLUAD QUADRIVALENT recipients and 18-28 (4.1%-6.3%) aTIV recipients experienced an SAE. There were no SAEs, AESIs or deaths in this study that were related to the study vaccine. There were no AEs leading to withdrawal from the study.
6.2 Postmarketing Experience
In addition to the adverse reactions observed during clinical trials, the following adverse events were reported from postmarketing surveillance in individuals 65 years of age and older for FLUAD QUADRIVALENT and/or for FLUAD (trivalent formulation), which is relevant because both vaccines are manufactured using the same process and have overlapping compositions.Because these events were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or to establish, for all events, a causal relationship to vaccine exposure.
Blood and lymphatic system disorders:
Thrombocytopenia (some cases were severe with platelet counts less than 5,000 per mm3), lymphadenopathy
Immune system disorders:
Allergic reactions including anaphylactic shock (in rare cases), anaphylaxis
Nervous system disorders:
Encephalomyelitis, Guillain-Barré Syndrome, convulsions, neuritis, neuralgia, paresthesia, syncope, presyncope, dizziness
Vascular disorders:
Vasculitis which may be associated with transient renal involvement
Skin and subcutaneous tissue disorders:
Generalized skin reactions including erythema multiforme, urticaria, pruritis or non-specific rash, erythema, angioedema
Musculoskeletal and connective tissue disorders:
Muscular weakness, pain in extremity
General disorders and administration site conditions:
Extensive swelling of injected limb, injection site cellulitis-like reaction, injection site swelling, peripheral swelling, asthenia, malaise, pyrexia
-
7 DRUG INTERACTIONS
7.1 Concomitant Use With Other Vaccines
No clinical data on concomitant administration of FLUAD QUADRIVALENT with other vaccines is available.
If FLUAD QUADRIVALENT is given at the same time as other injectable vaccine(s), the vaccine(s) should be administered at different injection sites.
Do not mix FLUAD QUADRIVALENT with any other vaccine in the same syringe.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
FLUAD QUADRIVALENT is not approved for use in persons < 65 years of age. There are insufficient human data to establish whether there is a vaccine-associated risk with use of FLUAD QUADRIVALENT in pregnancy.
There were no developmental toxicity studies of FLUAD QUADRIVALENT performed in animals. A developmental toxicity study has been performed in female rabbits administered FLUAD (trivalent formulation) prior to mating and during gestation. A 0.5 mL dose was injected on each occasion (a single human dose is 0.5 mL). (see 8.1 Animal Data).
Animal Data
In a developmental toxicity study, the effect of FLUAD (trivalent formulation) was evaluated in pregnant rabbits. Animals were administered FLUAD (trivalent formulation) by intramuscular injection twice prior to gestation, during the period of organogenesis (gestation day 7) and later in pregnancy (gestation day 20), 0.5 mL (45 mcg)/rabbit/occasion. No vaccine-related fetal malformations or variations and no adverse effects on pre-weaning development were observed in the study.
8.2 Lactation
FLUAD QUADRIVALENT is not approved for use in persons < 65 years of age. No human or animal data are available to assess the effects of FLUAD QUADRIVALENT on the breastfed infant or on milk production/excretion.
8.4 Pediatric Use
Safety and effectiveness of FLUAD and FLUAD QUADRIVALENT (same manufacturing process and overlapping composition with FLUAD) were evaluated in clinical trials conducted in children 6 months to <72 months of age. Data from these trials are inconclusive to demonstrate the safety and effectiveness of FLUAD QUADRIVALENT in children 6 months to <72 months of age. The safety and effectiveness of FLUAD QUADRIVALENT in infants less than 6 months of age and in children older than 72 months of age have not been evaluated.
-
11 DESCRIPTION
FLUAD QUADRIVALENT (Influenza Vaccine, Adjuvanted), a sterile injectable emulsion for intramuscular use, is a quadrivalent, inactivated influenza vaccine prepared from virus propagated in the allantoic cavity of embryonated hens' eggs inoculated with a specific type of influenza virus.
FLUAD QUADRIVALENT is standardized according to United States Public Health Service requirements and each 0.5 mL dose is formulated to contain 15 mcg of hemagglutinin (HA) from each of the following four influenza strains recommended for the 2023-2024 influenza season: A/Victoria/4897/2022 IVR-238 (an A/Victoria/4897/2022 (H1N1)pdm09-like virus), A/Darwin/6/2021 IVR-227 (an A/Darwin/9/2021 (H3N2)-like virus), B/Austria/1359417/2021 BVR-26 (a B/Austria/1359417/2021-like virus), B/Phuket/3073/2013 BVR-1B (a B/Phuket/3073/2013-like virus). FLUAD QUADRIVALENT also contains MF59C.1 adjuvant (MF59®), a squalene based oil-in-water emulsion. Each of the strains is harvested and clarified separately by centrifugation and filtration prior to inactivation with formaldehyde. The inactivated virus is concentrated and purified by zonal centrifugation. The surface antigens, hemagglutinin and neuraminidase, are obtained from the influenza virus particle by further centrifugation in the presence of cetyltrimethylammonium bromide (CTAB). The antigen preparation is further purified.
FLUAD QUADRIVALENT is prepared by combining the four virus antigens with the MF59C.1 adjuvant. After combining, FLUAD QUADRIVALENT is a sterile, milky-white injectable emulsion supplied in single-dose pre-filled syringes containing 0.5 mL dose. Each 0.5 mL dose contains 15 mcg of hemagglutinin (HA) from each of the four recommended influenza strains and MF59C.1 adjuvant (9.75 mg squalene, 1.175 mg of polysorbate 80, 1.175 mg of sorbitan trioleate, 0.66 mg of sodium citrate dihydrate and 0.04 mg of citric acid monohydrate) at pH 6.9-7.7.
FLUAD QUADRIVALENT may contain trace amounts of neomycin (≤ 0.02 mcg by calculation), kanamycin (≤ 0.03 mcg by calculation) and hydrocortisone (≤ 0.005 ng by calculation) which are used during the initial stages of manufacture, as well as residual egg protein (ovalbumin) (≤ 1.0 mcg), formaldehyde (≤ 10 mcg) or CTAB (≤ 18 mcg).
FLUAD QUADRIVALENT does not contain a preservative. The syringe, syringe plunger stopper and tip caps are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Influenza illness and its complications follow infection with influenza viruses. Global surveillance of influenza identifies yearly antigenic variants. For example, since 1977, antigenic variants of influenza A (H1N1 and H3N2) viruses and influenza B viruses have been in global circulation. Specific levels of hemagglutination inhibition (HI) antibody titers induced by vaccination with inactivated influenza virus vaccine have not been correlated with protection from influenza illness. In some human studies, HI antibody titers of 1:40 or greater have been associated with protection from influenza illness in up to 50% of subjects. [see References (2,3)]
Antibody against one influenza virus type or subtype confers limited or no protection against another. Furthermore, antibody to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype. Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual change of one or more new strains in each year's influenza vaccine. Therefore, inactivated quadrivalent influenza vaccines are standardized to contain the hemagglutinin of influenza virus strains (two subtypes A and two types B), representing the influenza viruses likely to be circulating in the United States in the upcoming influenza season.
Annual influenza vaccination is recommended because immunity declines during the year after vaccination, and because circulating strains of influenza virus change from year to year.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
FLUAD QUADRIVALENT has not been evaluated for carcinogenic or mutagenic potential, or for impairment of male fertility in animals. FLUAD (trivalent formulation) did not affect female fertility in a rabbit developmental toxicity study [see Pregnancy (8.1)].
-
14 CLINICAL STUDIES
14.1 Immunogenicity of FLUAD QUADRIVALENT
Immunogenicity of FLUAD QUADRIVALENT was evaluated in Study 1 (NCT02587221), a randomized, observer-blind, non-influenza comparator-controlled multicenter efficacy study conducted in 12 countries during the 2016-2017 Northern Hemisphere and 2017 Southern Hemisphere seasons. In this study, elderly subjects 65 years of age and older received one dose of either FLUAD QUADRIVALENT (N=3379) or a US-licensed non-influenza comparator vaccine (Boostrix; N=3382). Immunogenicity was evaluated 21 days after vaccination in a subgroup of subjects in a 4:1 ratio: FLUAD QUADRIVALENT (N=1324) and non-influenza control vaccines (N=332). In the immunogenicity set, the mean age across both vaccination groups was 72 years and females represented 59% of subjects. The racial distribution of subjects consisted of 89% Caucasian, 11% Asian and <1% American Indian or Alaska Native.
Immunogenicity endpoints measured 3 weeks after vaccination included percentage of subjects with HI titer ≥1:40 and percentage of subjects who achieved seroconversion. Success criteria required the lower bound of the 2-sided 95% CI for the proportion of subjects with an HI titer ≥1:40 to be ≥60% and for the lower bound of the 2-sided 95% CI for the proportion of subjects with seroconversion to be ≥30%. Antibody responses for all 4 strains are presented in Table 2.
Table 2: Immune Responses 21 Days After Vaccination with FLUAD QUADRIVALENT or a Non-Influenza Comparator Vaccine in Elderly Subjects 65 years of Age and Older (Study 1) Abbreviations: CI=Confidence Interval, N=number of subjects in full analysis immunogenicity set.
Non-Influenza Comparator Vaccine = combined Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Boostrix® (GlaxoSmithKline Biologicals)
aSuccess criteria: LB of the 95% CI for the % of subjects with HI titer ≥1:40 must be ≥60%
bSeroconversion is defined as a pre-vaccination HI titer <1:10 and post-vaccination HI titer ≥ 1:40 or at least a 4-fold increase in HI from pre-vaccination HI titer ≥ 1:10. Success criteria: the LB of the 95% CI for the SCR must be ≥30%.
Strain Proportion of subjects with HI titer ≥1:40a
(95% CI)
Fluad QUADRIVALENT
N=1324Proportion of subjects with HI titer ≥1:40a
(95% CI)
Non-Influenza Comparator Vaccine
N=332Seroconversionb
(95% CI)
Fluad QUADRIVALENT
N=1324Seroconversionb
(95% CI)
Non-Influenza Comparator Vaccine
N=332A/H1N1 96.2%
(95.1%, 97.2%)46.7%
(41.2%, 52.2%)78.0%
(75.7%, 80.2%)2.1%
(0.9%, 4.3%)A/H3N2 95.6%
(94.4%, 96.7%)41.7%
(36.3%, 47.2%)84.6%
(82.5%, 86.5%)3.9%
(2.1%, 6.6%)B/Yamagata 79.2%
(77.0%, 81.4%)21.5%
(17.2%, 26.4%)60.8%
(58.1%, 63.4%)3.6%
(1.9%, 6.3%)B/Victoria 81.6%
(79.4%, 83.7%)18.4%
(14.4%, 23.0%)65.5%
(62.9%, 68.1%)2.1%
(0.9%, 4.3%) -
15 REFERENCES
-
Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barre syndrome and the 1992-1993 and 1993-1994 influenza vaccines. N Engl J Med 1998; 339(25): 1797-1802.
-
Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004; 103:133-138.
-
Hobson D, Curry RL, Beare A, et. al. The role of serum hemagglutinin-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg Camb 1972; 767-777.
-
-
16 HOW SUPPLIED/STORAGE AND HANDLING
FLUAD QUADRIVALENT is supplied in the product presentation listed below:
Presentation Carton
NDC NumberComponents Pre-Filled Syringe 70461-123-03 0.5 mL dose in a pre-filled syringe (needle not supplied), package of 10 syringes per carton [NDC 70461-123-04] Store FLUAD QUADRIVALENT refrigerated at 2°C to 8°C (36°F to 46°F). Protect from light. Do not freeze. Discard if the vaccine has been frozen. Do not use after expiration date.
The syringe, syringe plunger stopper and tip cap are not made with natural rubber latex.
-
17 PATIENT COUNSELING INFORMATION
- Inform vaccine recipients of the potential benefits and risks of immunization with FLUAD QUADRIVALENT.
- Educate vaccine recipients regarding the potential side effects. Clinicians should emphasize that (1) FLUAD QUADRIVALENT contains non-infectious particles and cannot cause influenza and (2) FLUAD QUADRIVALENT is intended to help provide protection against illness due to influenza viruses only.
- Instruct vaccine recipients to report adverse reactions to their healthcare provider and/or to Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 and www.vaers.hhs.gov. Provide vaccine recipients with the Vaccine Information Statements which are required by the National Childhood Vaccine Injury Act of 1986. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
- Inform vaccine recipients that annual vaccination is recommended.
FLUAD QUADRIVALENT is a registered trademark of Seqirus UK Limited or its affiliates.
MF59® is a trademark of Novartis AG.
Manufactured by: Seqirus Inc., 475 Green Oaks Parkway, Holly Springs, NC 27540, USA
Distributed by: Seqirus USA Inc., 25 Deforest Avenue, Summit, NJ 07901, USA
Tel: 1-855-358-8966
US License No. 2049
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLUAD QUADRIVALENT
influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/darwin/6/2021 ivr-227 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/austria/1359417/2021 bvr-26 antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 bvr-1b antigen (formaldehyde inactivated) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:70461-123 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: AU5C98U4BB) (INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:C46XJT9FQ9) INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 15 ug in 0.5 mL INFLUENZA A VIRUS A/DARWIN/6/2021 IVR-227 (H3N2) ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: F5QV7AF326) (INFLUENZA A VIRUS A/DARWIN/6/2021 IVR-227 (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:N969QK7XD2) INFLUENZA A VIRUS A/DARWIN/6/2021 IVR-227 (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 15 ug in 0.5 mL INFLUENZA B VIRUS B/AUSTRIA/1359417/2021 BVR-26 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: TZK7Q3545Q) (INFLUENZA B VIRUS B/AUSTRIA/1359417/2021 BVR-26 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:V4C2RVJ2EY) INFLUENZA B VIRUS B/AUSTRIA/1359417/2021 BVR-26 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 15 ug in 0.5 mL INFLUENZA B VIRUS B/PHUKET/3073/2013 BVR-1B ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: G1T4TD4PZC) (INFLUENZA B VIRUS B/PHUKET/3073/2013 BVR-1B HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:9YRA9J0KI2) INFLUENZA B VIRUS B/PHUKET/3073/2013 BVR-1B HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 15 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) potassium chloride (UNII: 660YQ98I10) potassium phosphate, monobasic (UNII: 4J9FJ0HL51) sodium phosphate, dibasic, dihydrate (UNII: 94255I6E2T) magnesium chloride (UNII: 02F3473H9O) calcium chloride (UNII: M4I0D6VV5M) citric acid monohydrate (UNII: 2968PHW8QP) sorbitan trioleate (UNII: QE6F49RPJ1) trisodium Citrate dihydrate (UNII: B22547B95K) water (UNII: 059QF0KO0R) squalene (UNII: 7QWM220FJH) polysorbate 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70461-123-03 10 in 1 CARTON 1 NDC:70461-123-04 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125510 07/01/2023 07/31/2024 Labeler - Seqirus, Inc. (080102141)