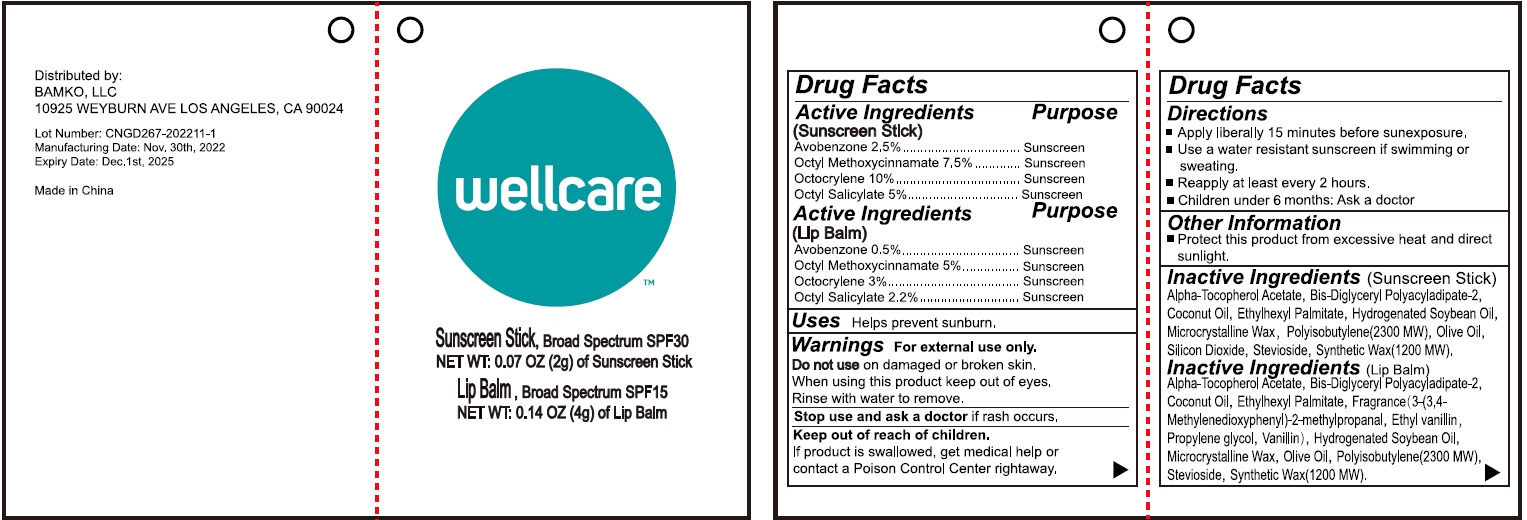
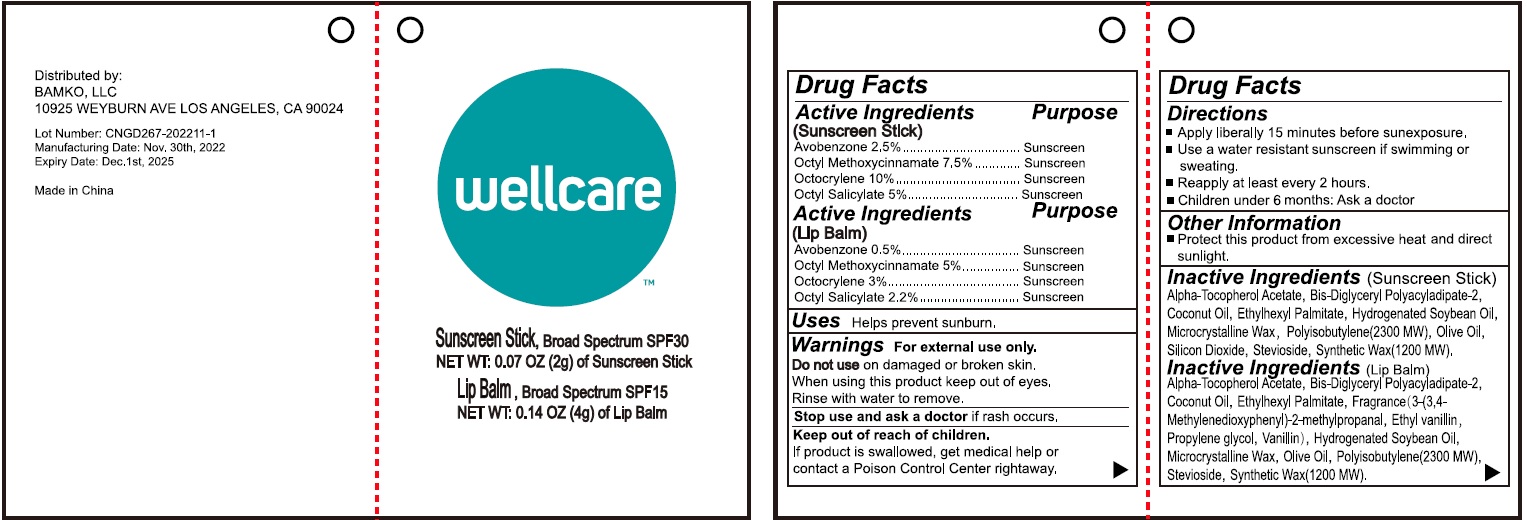
Label: WELLCARE SUNSTICK SUNSCREEN STICK- avobenzone, octinoxate, octocrylene, octisalate stick
WELLCARE SUNSTICK LIP BALM- avobenzone, octinoxate, octocrylene, octisalate stick
- NDC Code(s): 83149-000-00, 83149-001-00
- Packager: BAMKO, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
-
Inactive Ingredients
Alpha-Tocopherol Acetate, Bis-Diglyceryl Polyacyladipate-2, Coconut Oil, Ethylhexyl Palmitate, Fragrance (3-(3,4-Methylenedioxyphenyl)-2-methylpropanal, Ethyl vanillin, Propylene glycol, Vanillin), Hydrogenated Soybean Oil, Microcrystalline Wax, Olive Oil, Polyisobutylene(2300 MW), Stevioside, Synthetic Wax (1200 MW).
- Wellcare Sunstick - Lip Balm, 0.14oz/4g (83149-000-00)
- Wellcare Sunstick - Sunscreen Stick, 0.07oz/2g (83149-001-00)
-
INGREDIENTS AND APPEARANCE
WELLCARE SUNSTICK SUNSCREEN STICK
avobenzone, octinoxate, octocrylene, octisalate stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83149-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 25 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) COCONUT OIL (UNII: Q9L0O73W7L) ETHYLHEXYL PALMITATE (UNII: 2865993309) ETHYL VANILLIN (UNII: YC9ST449YJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) VANILLIN (UNII: CHI530446X) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) OLIVE OIL (UNII: 6UYK2W1W1E) POLYISOBUTYLENE (2300 MW) (UNII: DSQ2V1DD1K) STEVIOSIDE (UNII: 0YON5MXJ9P) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83149-001-00 2 g in 1 TUBE; Type 0: Not a Combination Product 12/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/15/2022 WELLCARE SUNSTICK LIP BALM
avobenzone, octinoxate, octocrylene, octisalate stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83149-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 5 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 50 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 30 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 22 mg in 1 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) COCONUT OIL (UNII: Q9L0O73W7L) ETHYLHEXYL PALMITATE (UNII: 2865993309) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) POLYISOBUTYLENE (2300 MW) (UNII: DSQ2V1DD1K) OLIVE OIL (UNII: 6UYK2W1W1E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEVIOSIDE (UNII: 0YON5MXJ9P) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83149-000-00 4 g in 1 TUBE; Type 0: Not a Combination Product 12/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/15/2022 Labeler - BAMKO, LLC (080220012)