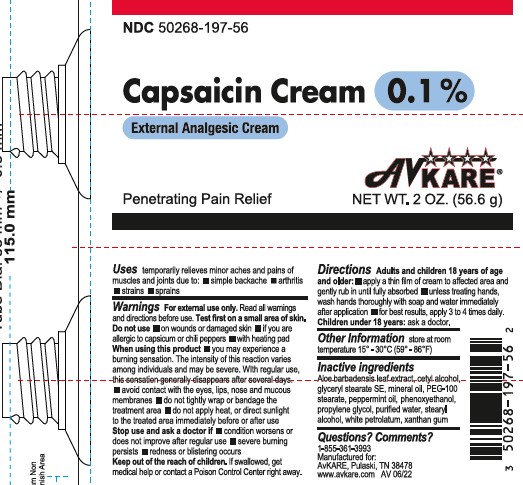
Label: CAPSAICIN cream
- NDC Code(s): 50268-197-56
- Packager: AvPAK
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings: For external use only.
Do not use:
- on wounds or damaged skin
- if you are allergic to capsicum or chili peppers
- with heating pad
When using this produce:
- you may experience a burning sensation The intensity of this reaction varies among individuals and may be severe. With regular use, the sensation generally disappears after several days.
- avoid contact with the eyes, lips, nose and mucous membranes
- do not tightly wrap or bandage the treatement area
- do not apply heat, or direct sunlight to the treated area immediately before or after use
Stop use and ask a doctor if:
- condition worsens or does not improve after regular use
- severe burning persists
- redness or blistering occurs
-
DOSAGE & ADMINISTRATION
Directions – Adults and children 18 years of age and older
- apply a thin film of cream to affected area and gently rub in until fully absorbed
- unless treating hands, wash hands thoroughly with soap and water immediately after application
- for best results, apply 3 to 4 times daily
Children under 18 years: ask a doctor. - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
CAPSAICIN
capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50268-197 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.001 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETYL ALCOHOL (UNII: 936JST6JCN) PETROLATUM (UNII: 4T6H12BN9U) PEPPERMINT OIL (UNII: AV092KU4JH) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) XANTHAN GUM (UNII: TTV12P4NEE) MINERAL OIL (UNII: T5L8T28FGP) ALOE VERA LEAF (UNII: ZY81Z83H0X) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50268-197-56 1 in 1 CARTON 12/08/2022 1 56.6 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/08/2022 Labeler - AvPAK (832926666)