Label: FLUNAZINE- flunixin meglumine paste
- NDC Code(s): 61133-6007-1
- Packager: Bimeda Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated March 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
INDICATIONS & USAGE
INDICATIONS: Flunazine Equine Paste is recommended for the alleviation of inflammation and pain associated with musculoskeletal disorders in the horse.
ACTIVITY: Flunixin meglumine is a potent, nonnarcotic, nonsteroidal, analgesic agent with anti-inflammatory and antipyretic activity. It is significantly more potent than pentazocine, meperidine, and codeine as an analgesic in the rat yeast paw test. Oral studies in the horse show onset of flunixin activity occurs within 2 hours of administration. Peak response occurs between 12 and 16 hours and duration of activity is 24 to 36 hours.
-
WARNINGS AND PRECAUTIONS
For oral use in horses only
CONTRAINDICATIONS: There are no known contraindications to this drug when used as directed.
WARNING: Do not use in horses intended for human consumption.
PRECAUTIONS: The effect of flunixin meglumine on pregnancy has not been determined. Studies to date show there is no detrimental effect on stallion spermatogenesis with or following the recommended dose of flunixin meglumine.
SIDE EFFECTS: During field studies with flunixin meglumine, no significant side effects were reported.To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VET or online at www.fda.gov/reportanimalae
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: The recommended dose of flunixin is 0.5 mg per lb of body weight once daily. The Flunazine Equine Paste syringe, calibrated in twelve 250-lb weight increments, delivers 125 mg of flunixin for each 250 lbs (see dosage table). One syringe will treat a 1000-lb horse once daily for 3 days, or three 1000-lb horses one time.
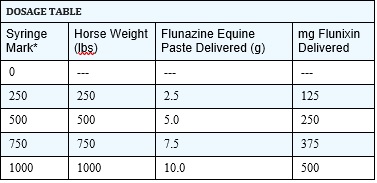
* Use dial edge nearest syringe barrel to mark dose.
The paste is orally administered by inserting the nozzle of the syringe through the interdental space, and depositing the required amount of paste on the back of the tongue by depressing the plunger.
Treatment may be given initially by intravenous or intramuscular injection of Flunazine Injectable Solution, followed by Flunazine Equine Paste on Days 2 to 5. Flunixin meglumine treatment should not exceed 5 consecutive days.
TOXICITY: No toxic effects were observed in rats given oral flunixin meglumine 2 mg/kg per day for 42 days. Higher doses produced ulceration of the gastrointestinal tract. The emetic dose in dogs is between 150 and 250 mg/kg. Flunixin was well tolerated in monkeys dosed daily with 4 mg/kg for 56 days. No adverse effects occurred in horses dosed orally with 1.0 or 1.5 mg/lb for 5 consecutive days.
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLUNAZINE
flunixin meglumine pasteProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:61133-6007 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUNIXIN MEGLUMINE (UNII: 8Y3JK0JW3U) (FLUNIXIN - UNII:356IB1O400) FLUNIXIN MEGLUMINE 1500 mg in 30 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61133-6007-1 30 g in 1 SYRINGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200581 02/26/2015 Labeler - Bimeda Inc. (060492923) Registrant - Bimeda Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda-MTC Animal Health 256232216 manufacture


