Label: KARANJA TREE SUNSHIELD SPF 30 MOLTON BROWN SKIN CARE- avobenzone, ensulizole, octinoxate, octocrylene cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 68814-128-01, 68814-128-02 - Packager: Molton Brown LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 4, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
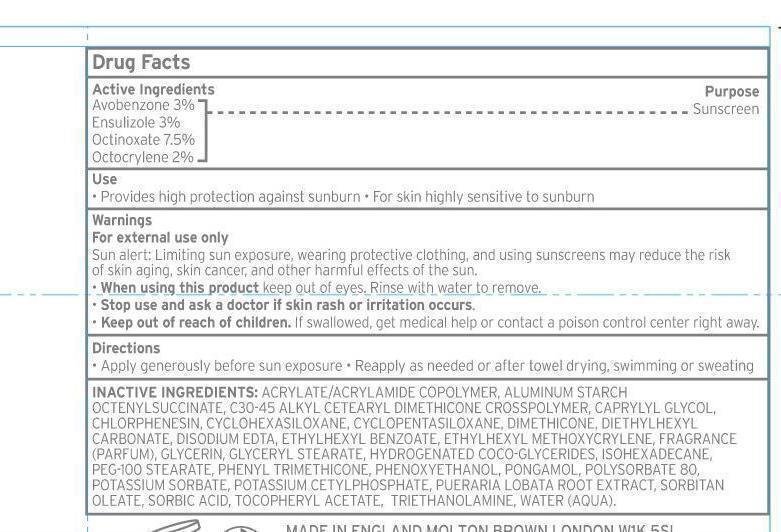
Active ingredients Purpose
Avobenzone 3% Sunscreen
Ensulizole 3% Sunscreen
Octinoxate 7.5% Sunscreen
Octocrylene 2% Sunscreen
keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
Warnings
For external use only.
Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risk of skin aging, skin cancer and other harmful effects of the sun.
When using this product, keep out of eyes. Rinse with water to remove.
Directions
-Apply generously before sun exposure
-reapply as needed or after towel drying, swimming, or sweating.
Inactive ingredients
Acrylate/acrylamide copolymer, aluminum starch octenylsuccinate, C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl glycol, chlorphenesin, cyclohexasiloxane, cyclopentasiloxane, dimethicone, diethylhexyl carbonate, disodium EDTA, ethylhexyl benzoate, ethyhexyl methoxycrylene,fragrance (parfum), glycerin, glyceryl stearate, hydrogenated coco-glycerides, isohexadecane, Peg-100 stearate, phenyl trimethicone, phenoxyethanol, pongamal, polysorbate 80, potassium sorbate, potassium cetylphosphate, Pueraria Lobata root extract, Sorbitan oleate, sorbic acid, Tocopheryl acetate, triethanolamine, water (aqua).
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KARANJA TREE SUNSHIELD SPF 30 MOLTON BROWN SKIN CARE
avobenzone, ensulizole, octinoxate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68814-128 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL ENSULIZOLE (UNII: 9YQ9DI1W42) (ENSULIZOLE - UNII:9YQ9DI1W42) ENSULIZOLE 3 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DIETHYLHEXYL CARBONATE (UNII: YCD50O0Z6L) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOHEXADECANE (UNII: 918X1OUF1E) POLYOXYL 100 STEARATE (UNII: YD01N1999R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) SORBIC ACID (UNII: X045WJ989B) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68814-128-02 1 in 1 BOX 1 NDC:68814-128-01 50 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/30/2010 Labeler - Molton Brown LTD (769334822) Registrant - Molton Brown LTD (769334822) Establishment Name Address ID/FEI Business Operations DDD Ltd. 216339804 manufacture(68814-128)