Label: FENTANYL patch
-
NDC Code(s):
59490-4190-9,
59490-4191-9,
59490-4192-9,
59490-4193-9, view more59490-4194-9
- Packager: Mylan Technologies Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: Export only
Drug Label Information
Updated January 22, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
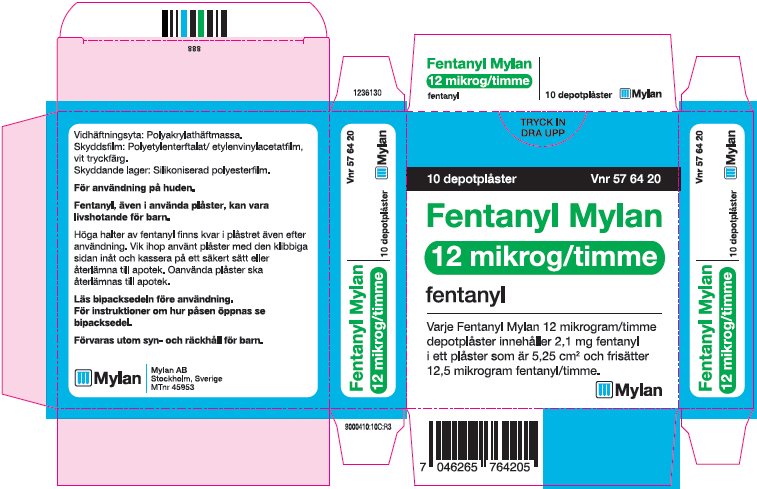
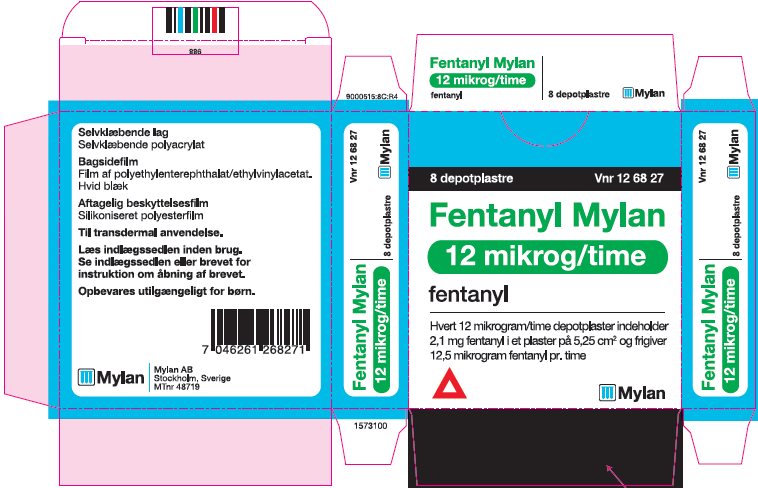
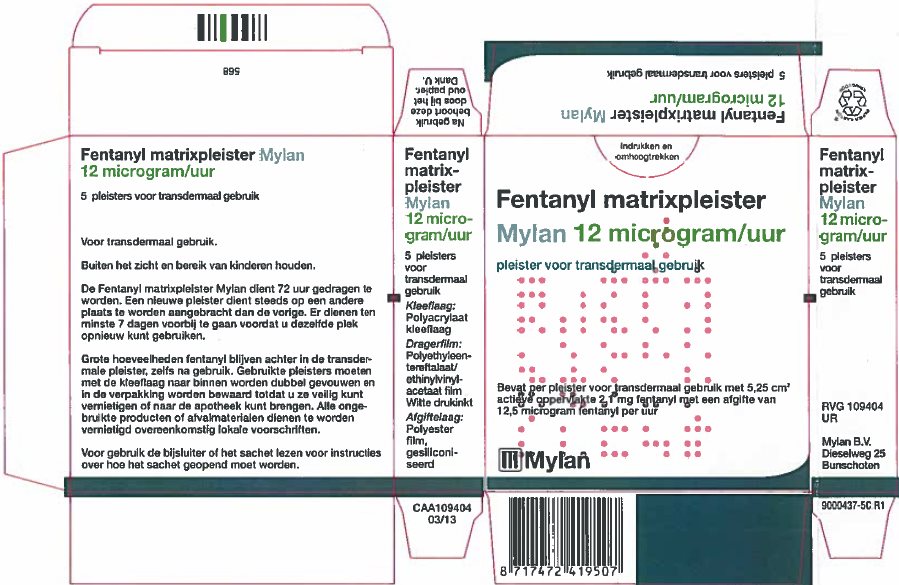
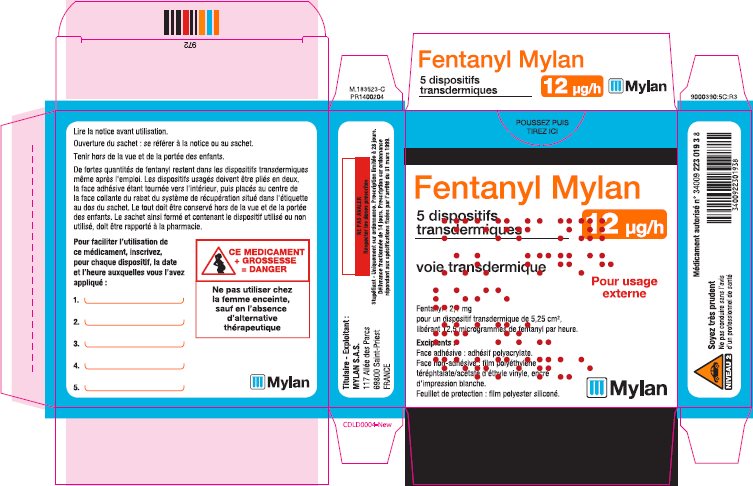
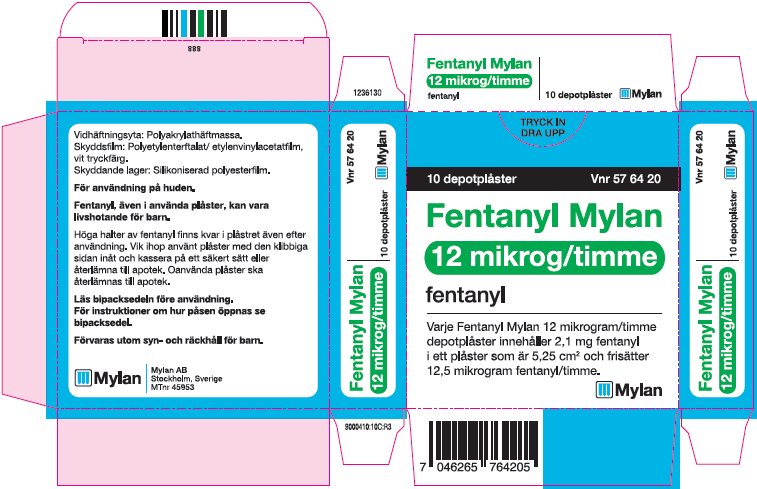
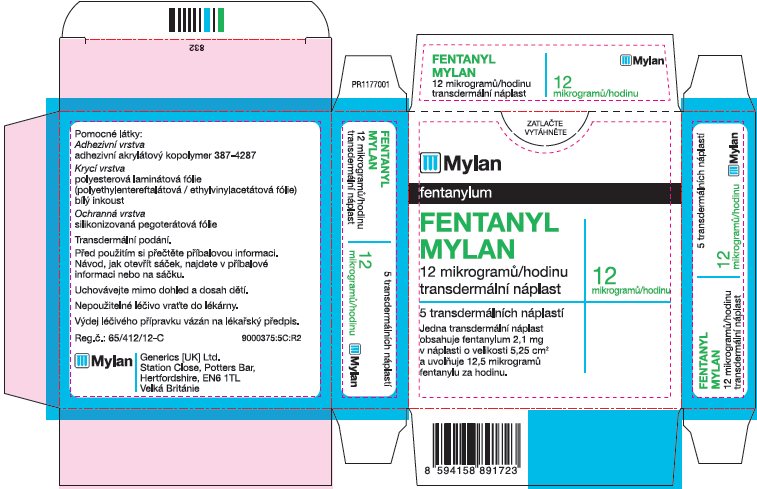
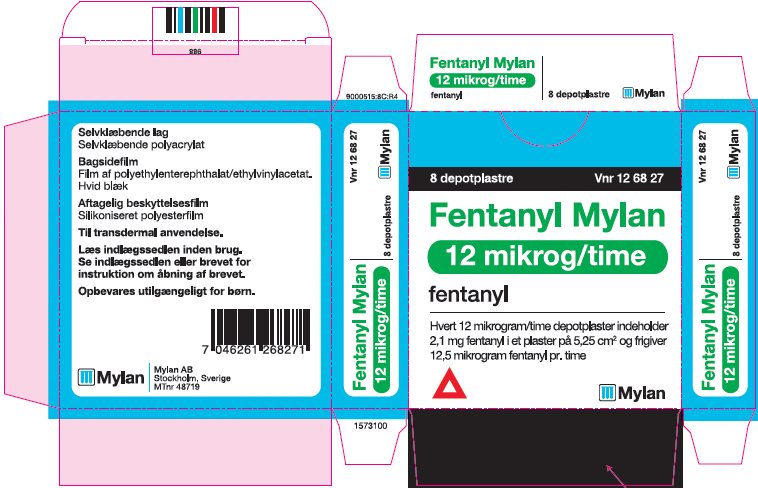
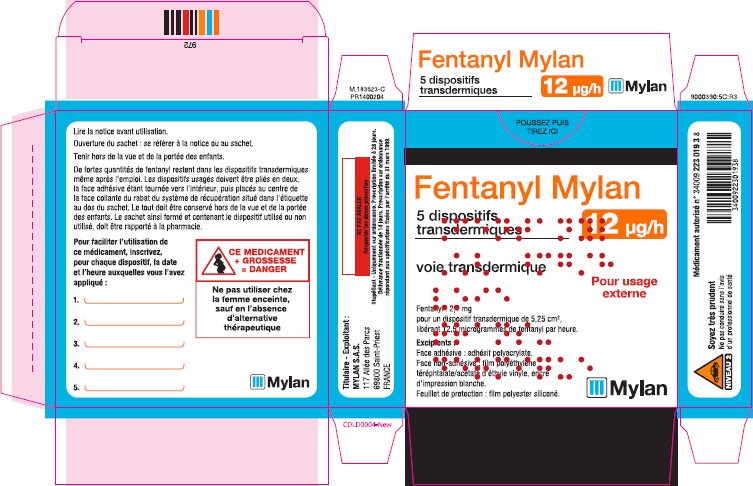
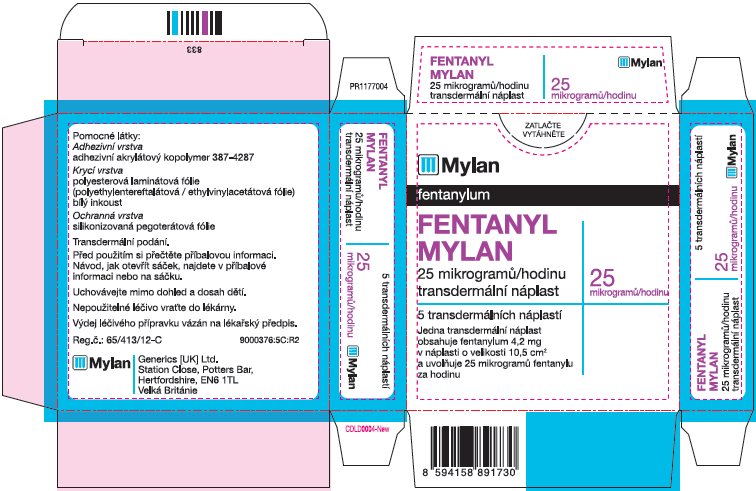
- PRINCIPAL DISPLAY PANEL – 12 microgram/hour
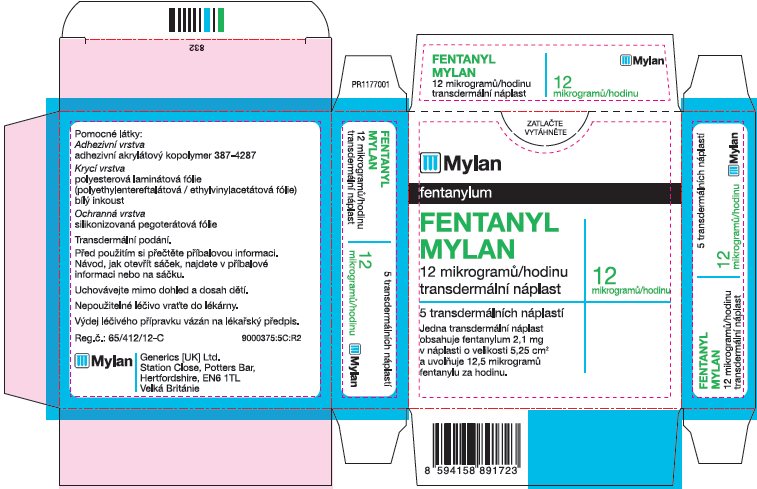
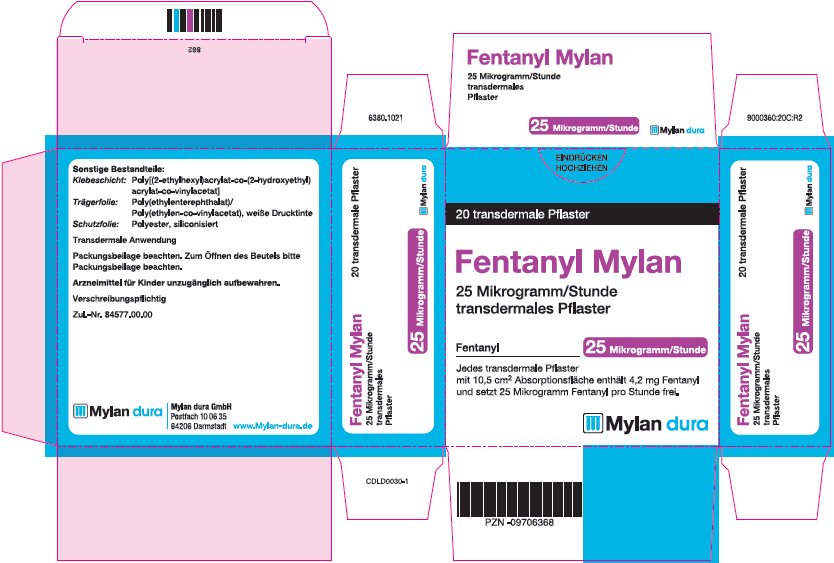
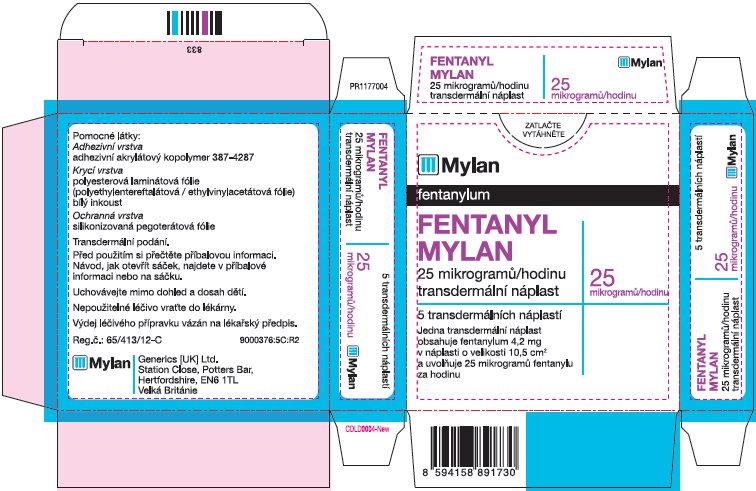
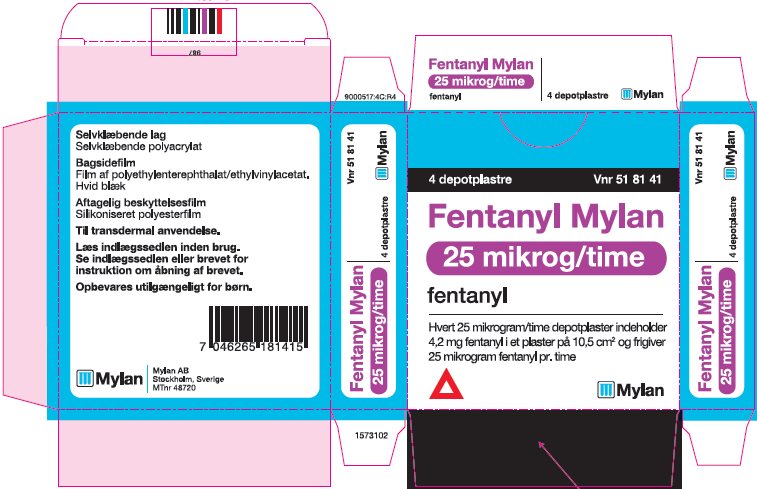
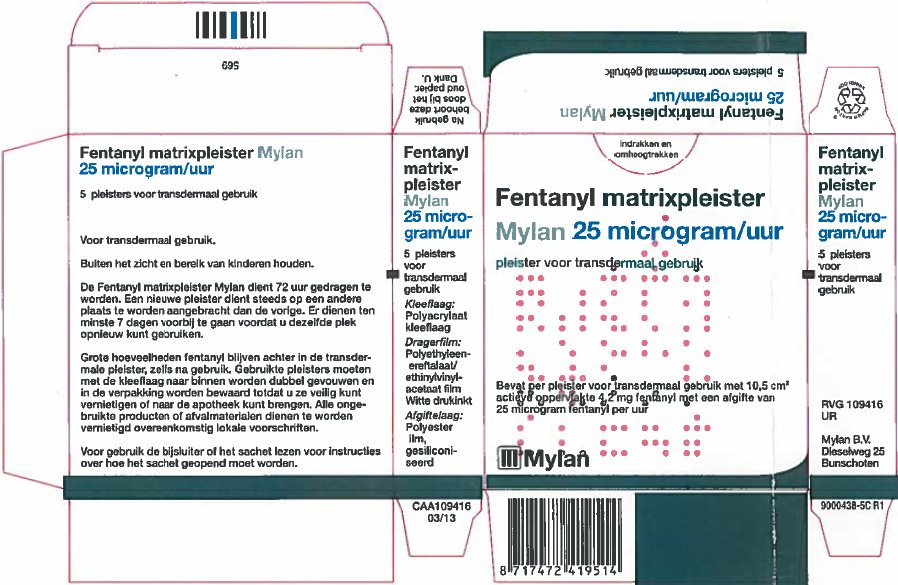
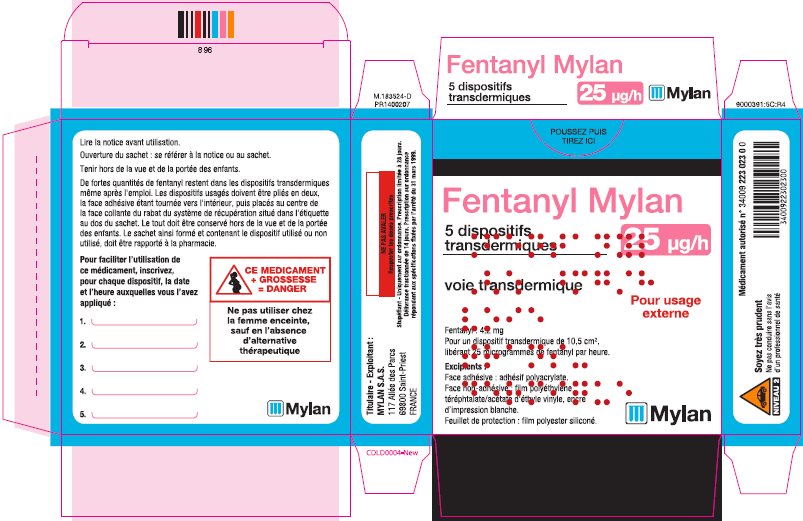
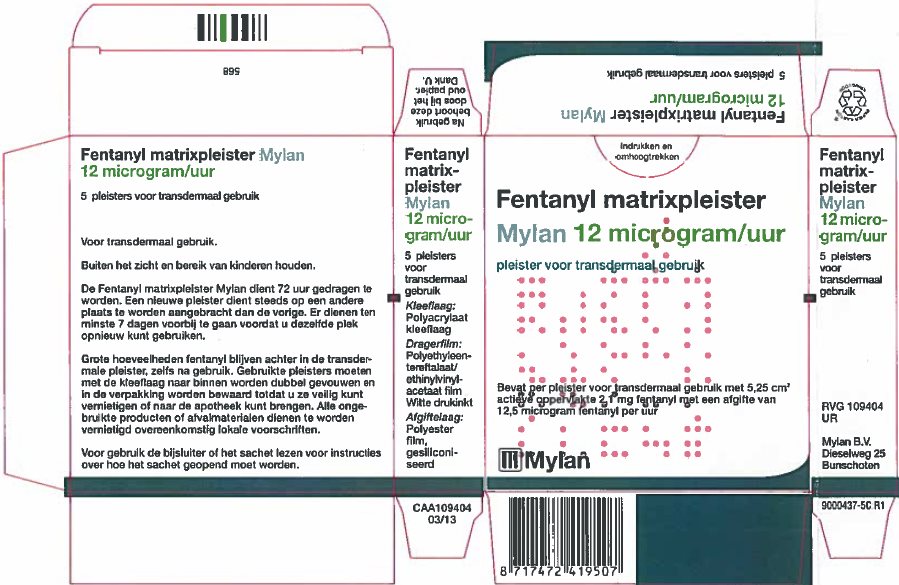
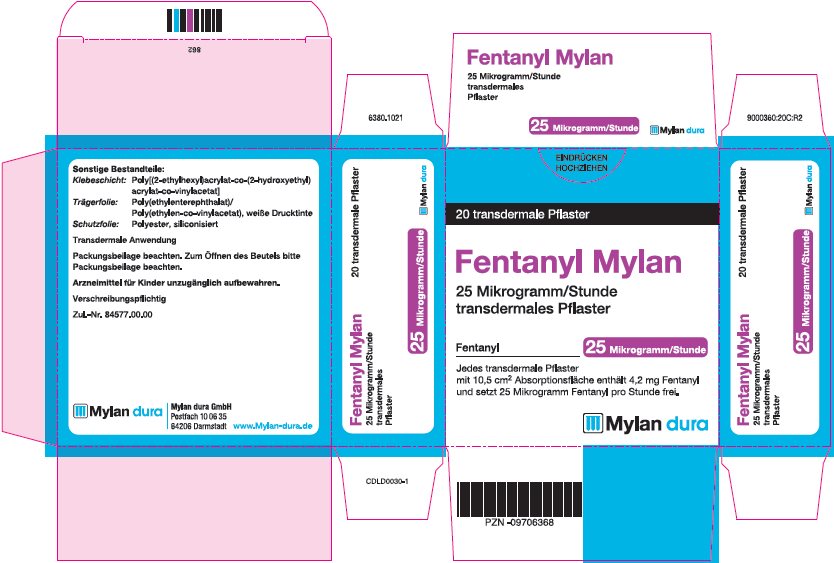
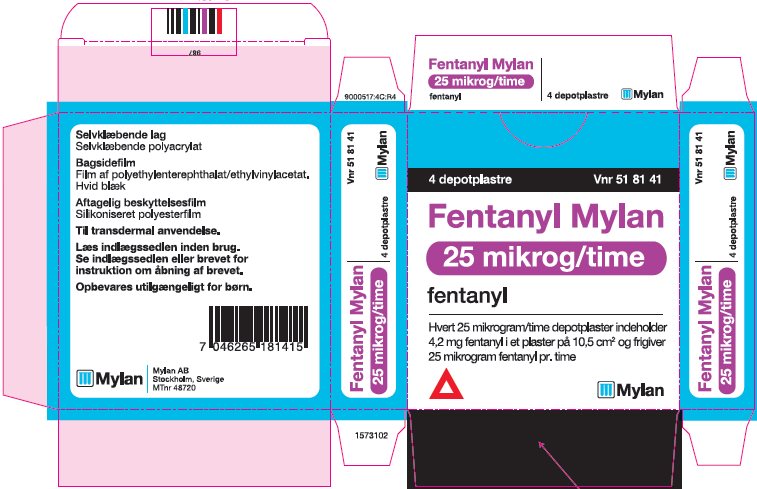
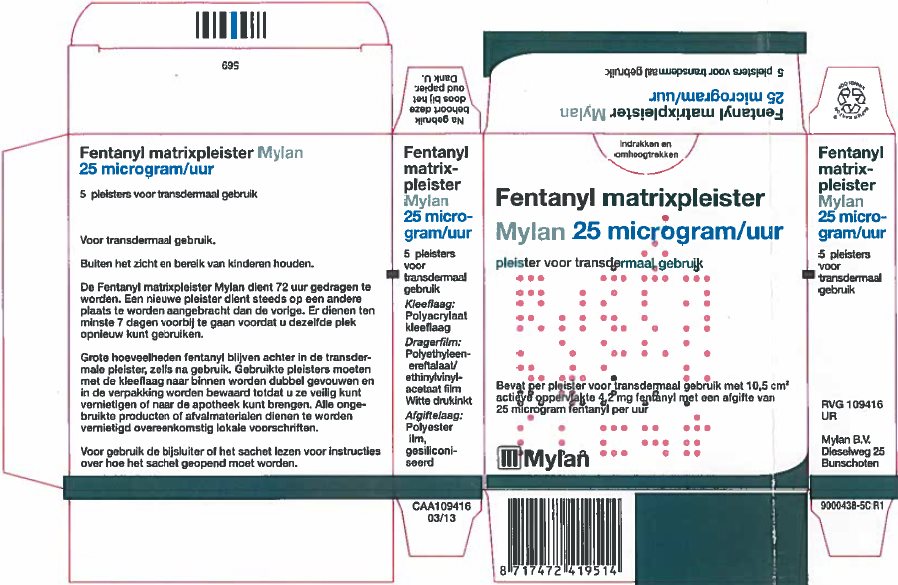
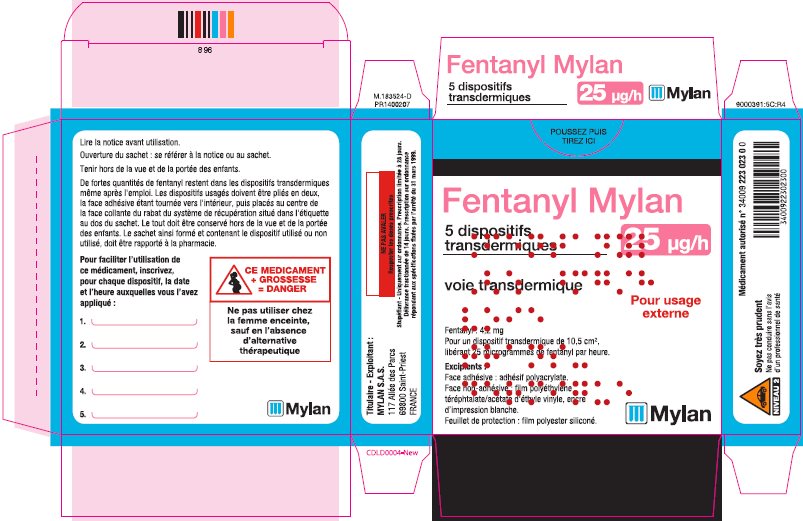
- PRINCIPAL DISPLAY PANEL – 25 microgram/hour
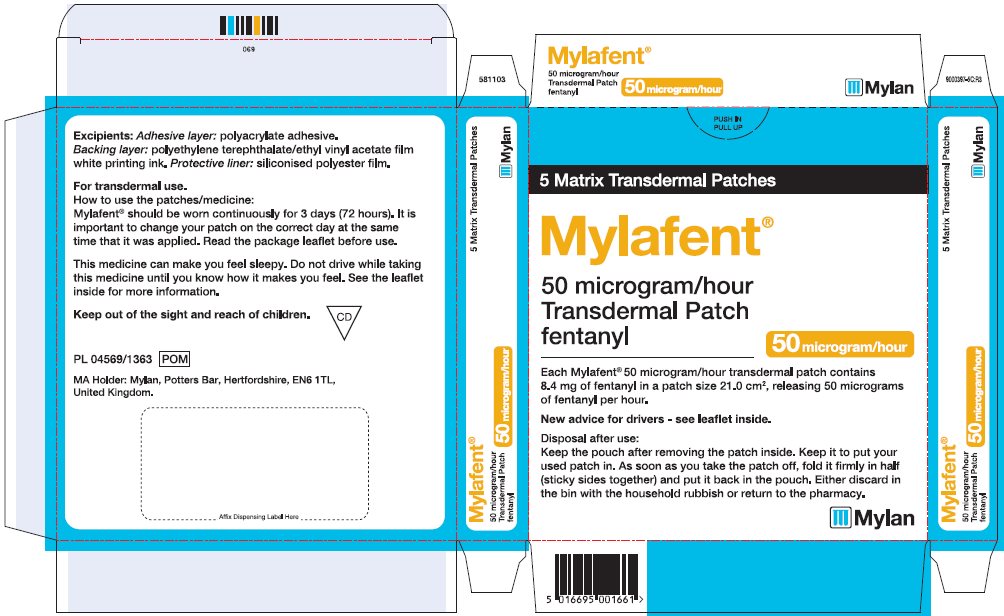
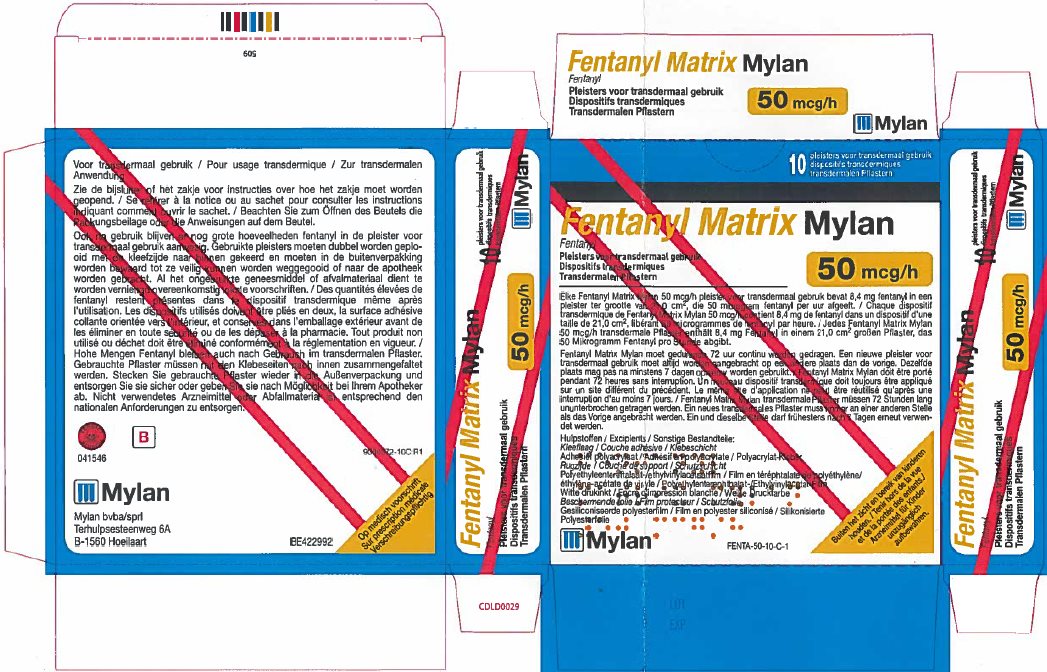
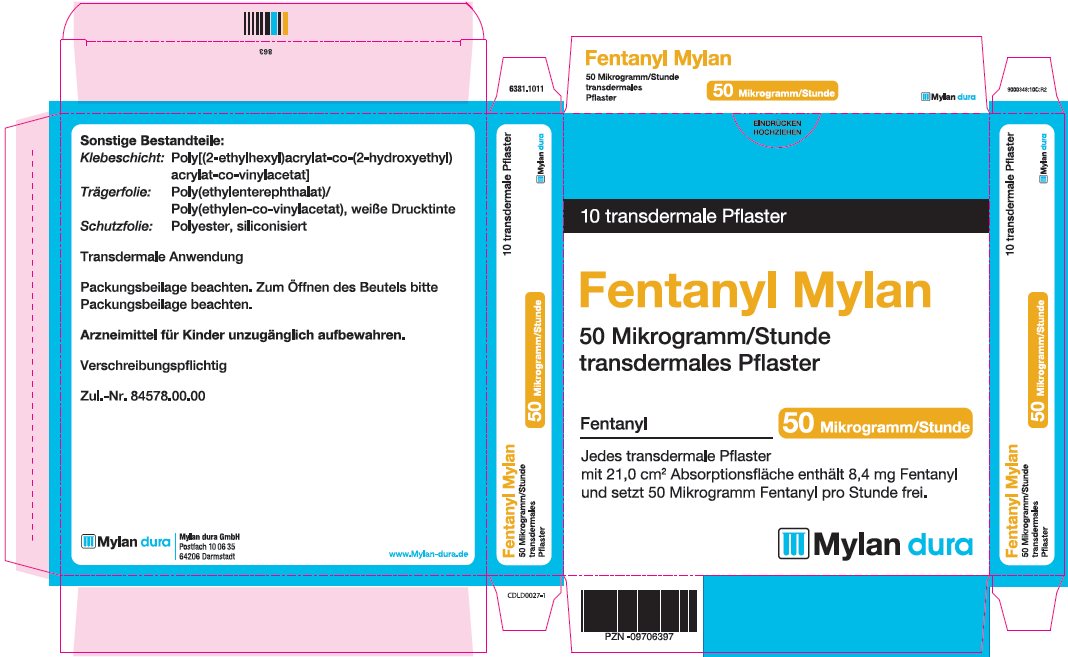
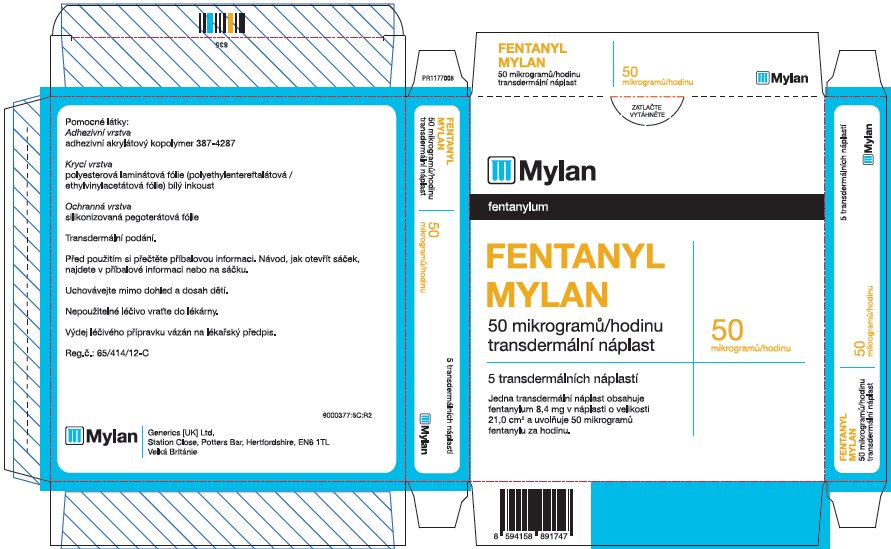
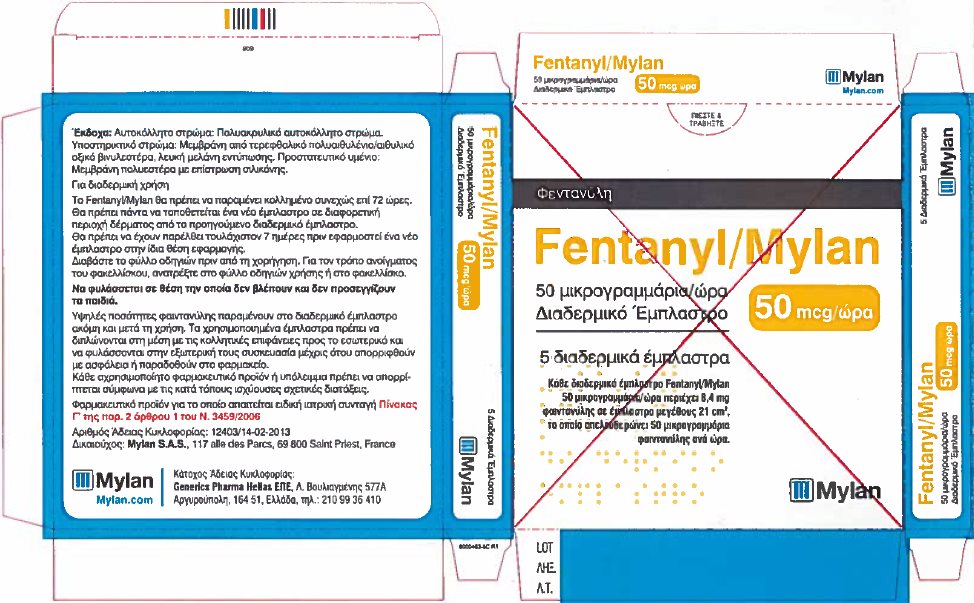
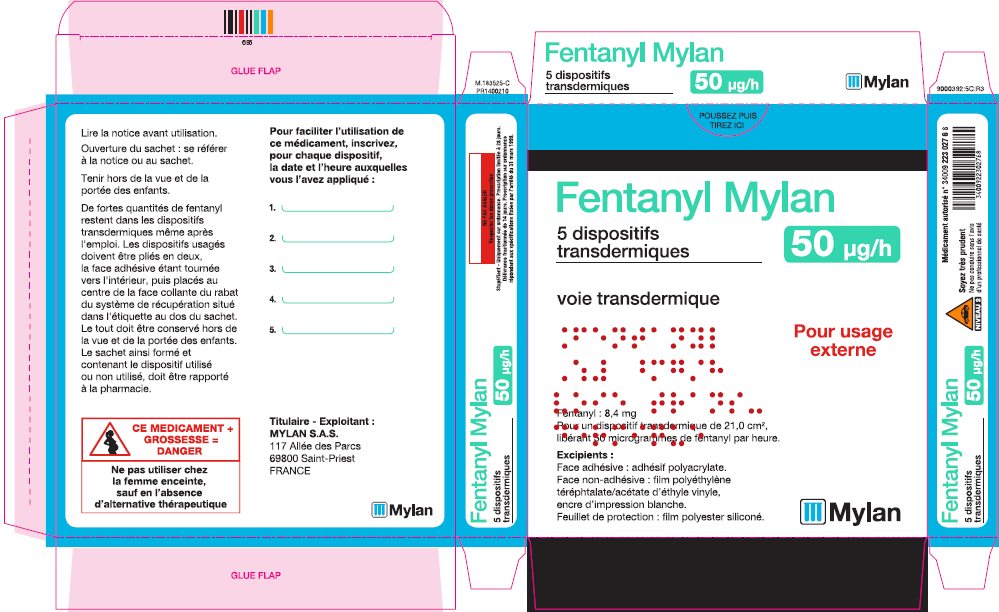
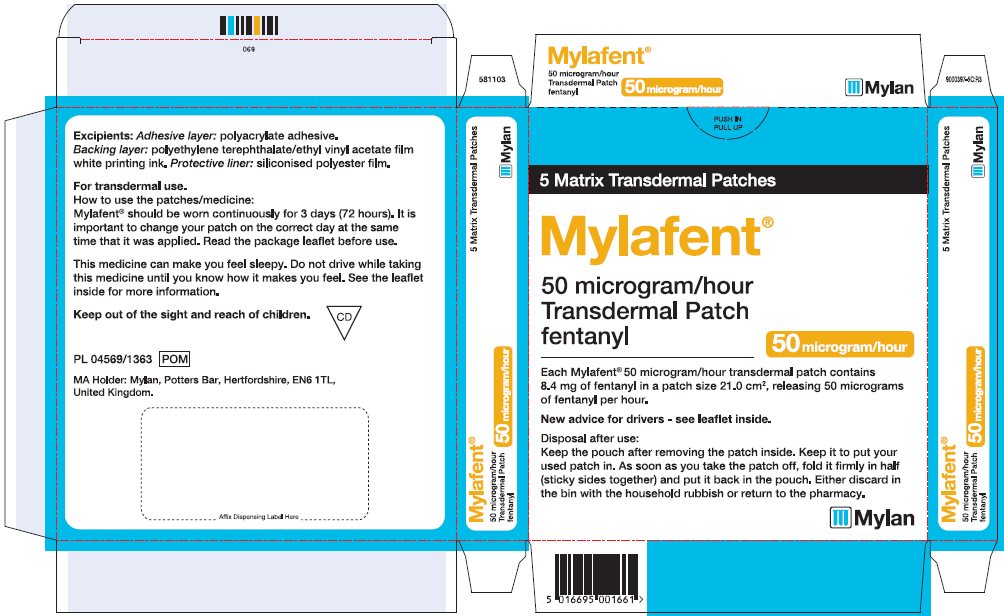
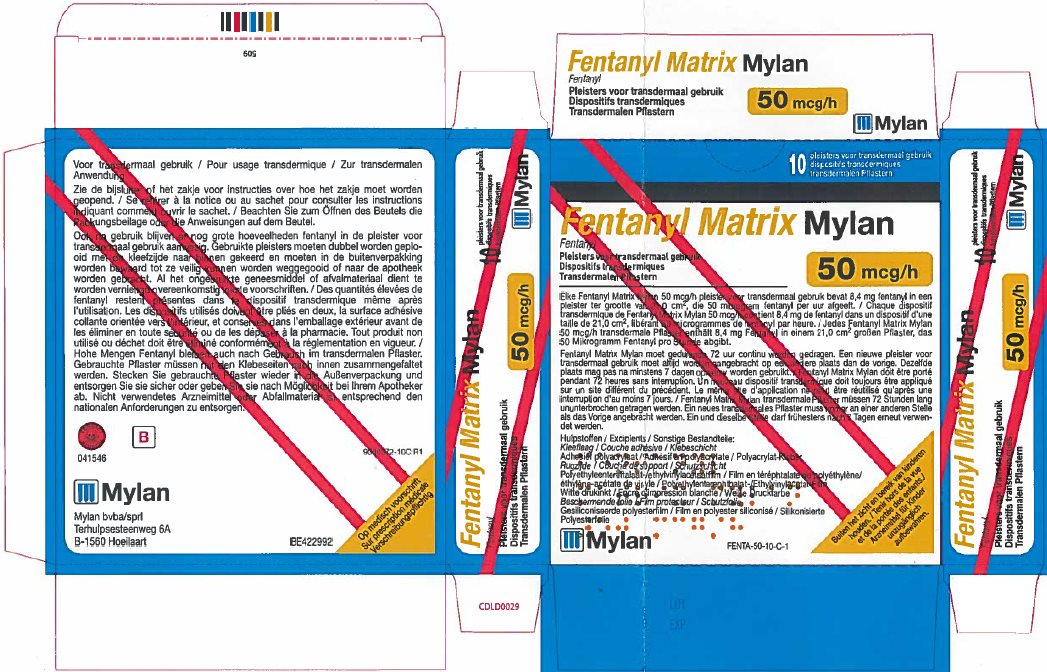
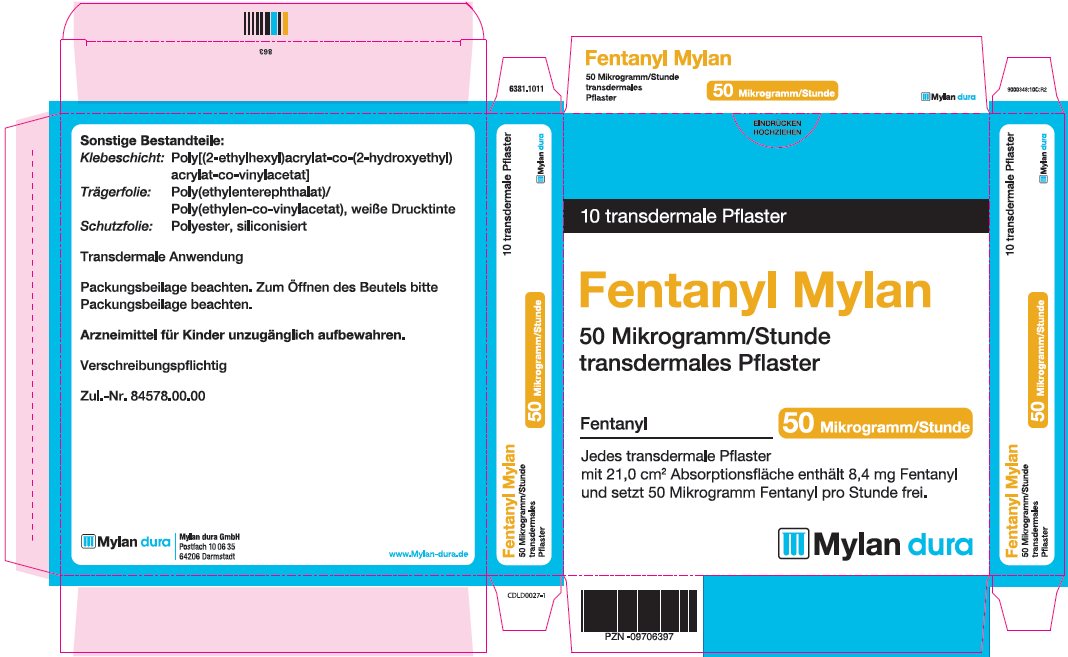
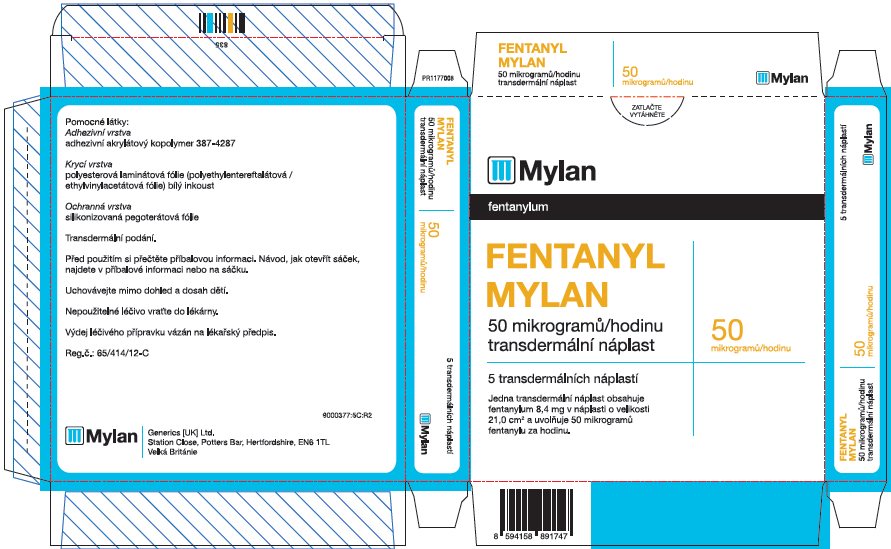
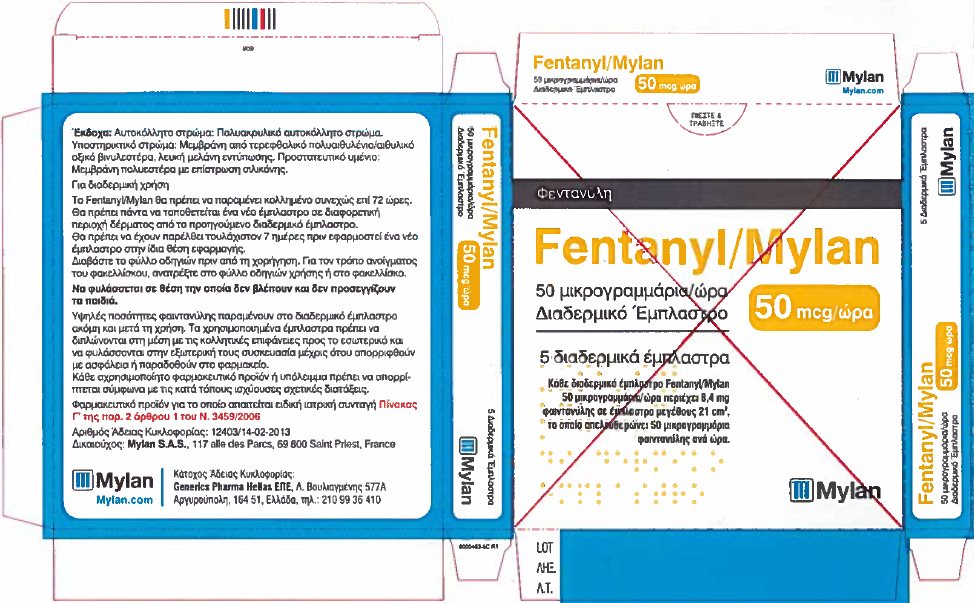
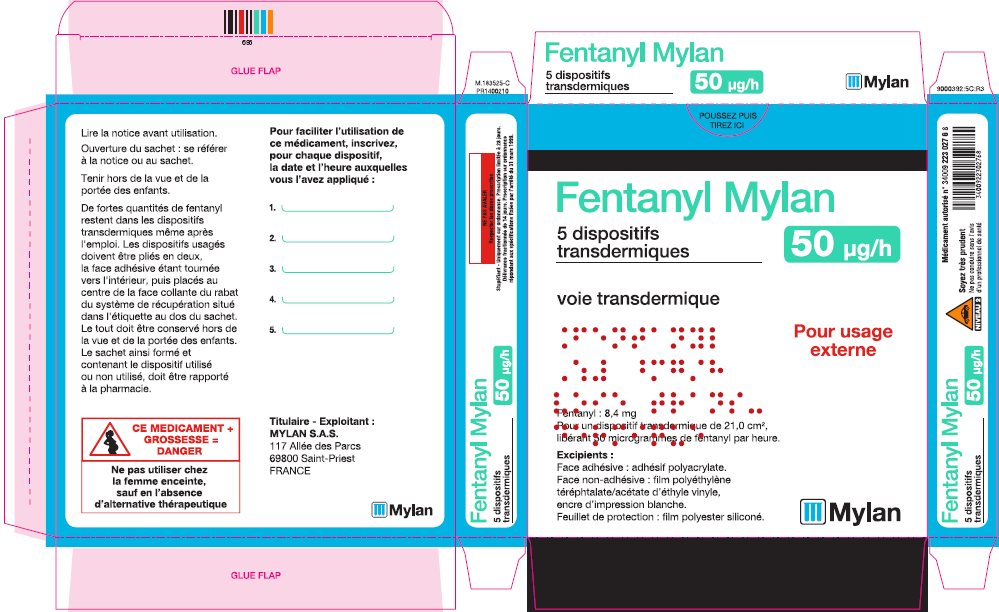
- PRINCIPAL DISPLAY PANEL – 50 microgram/hour
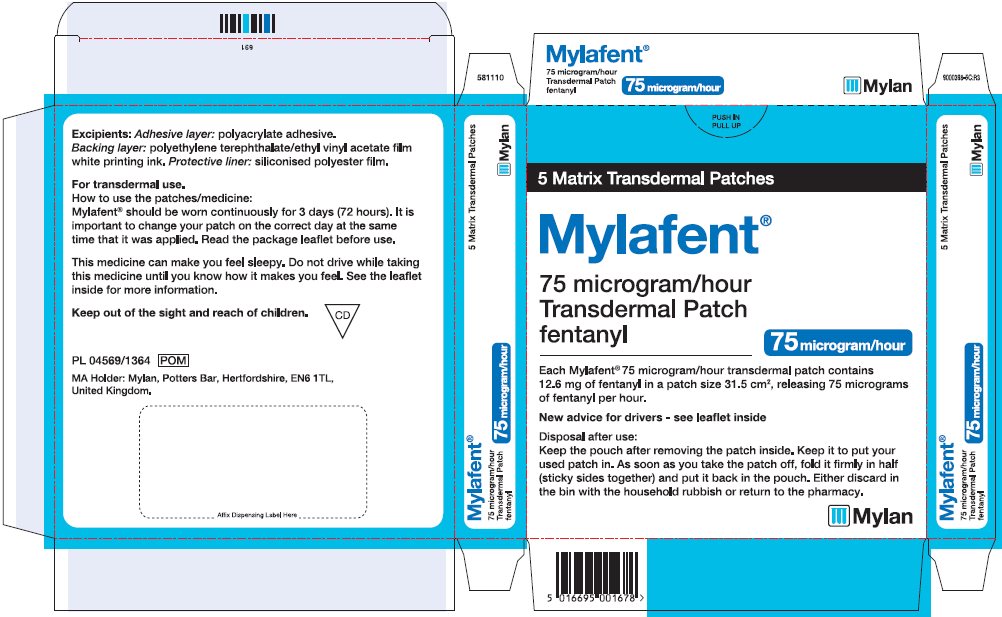
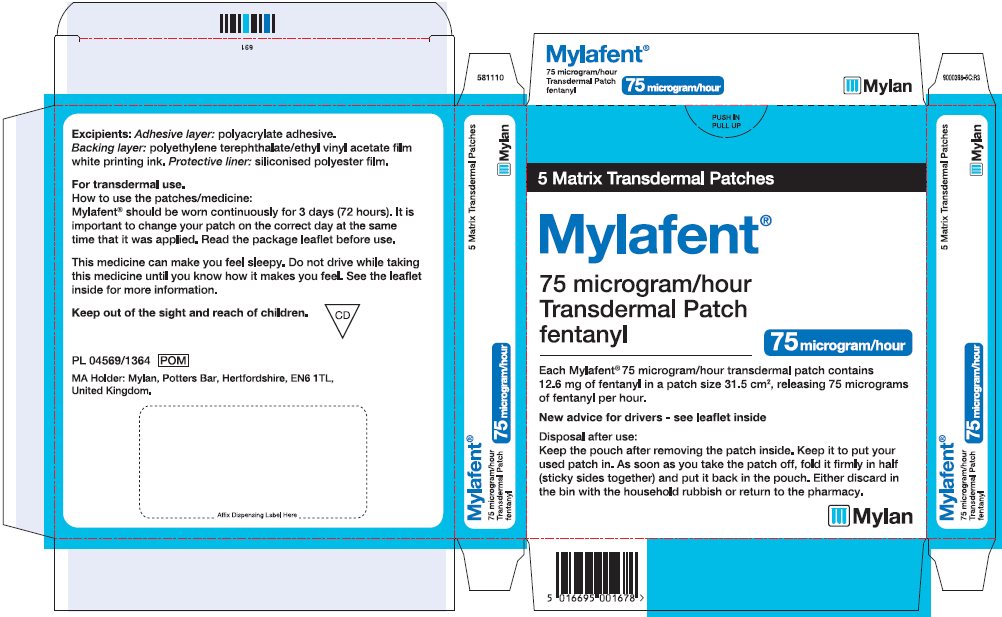
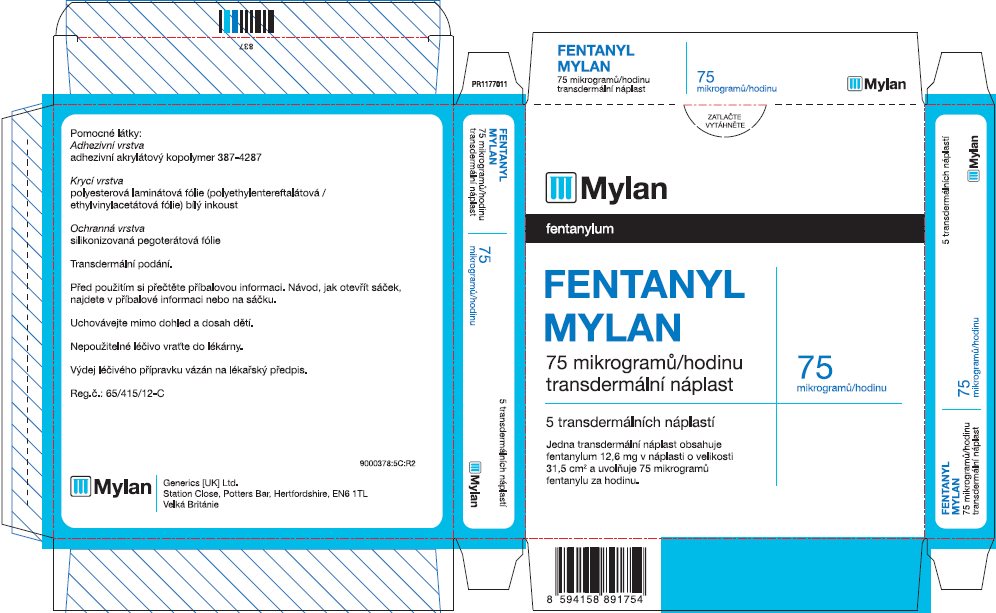
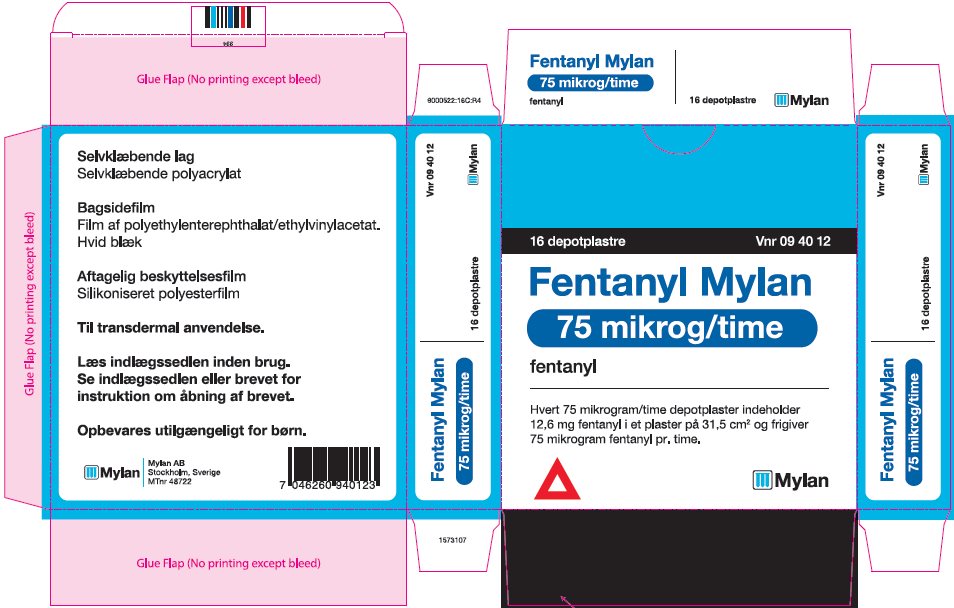
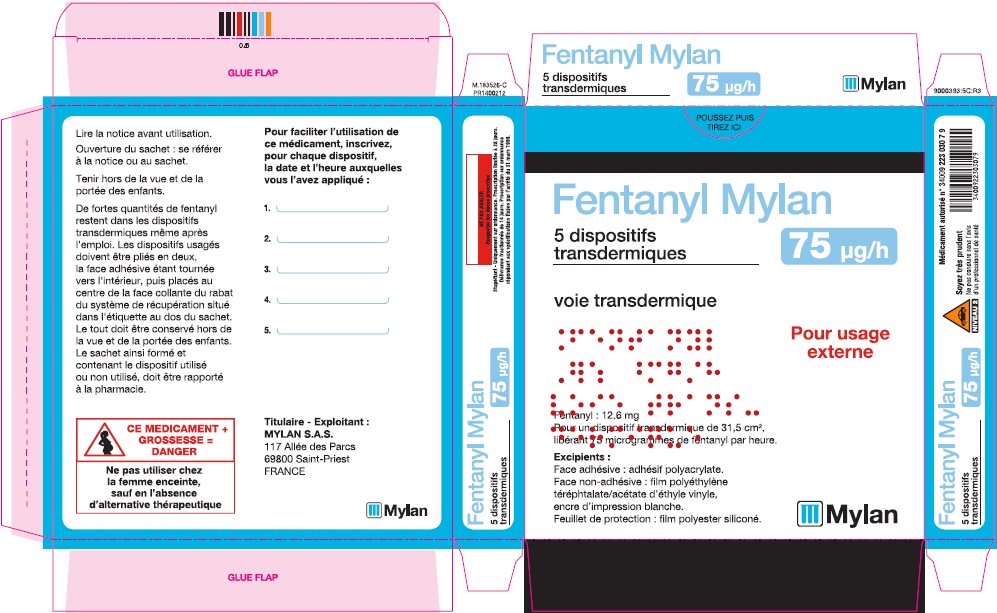
- PRINCIPAL DISPLAY PANEL – 75 microgram/hour
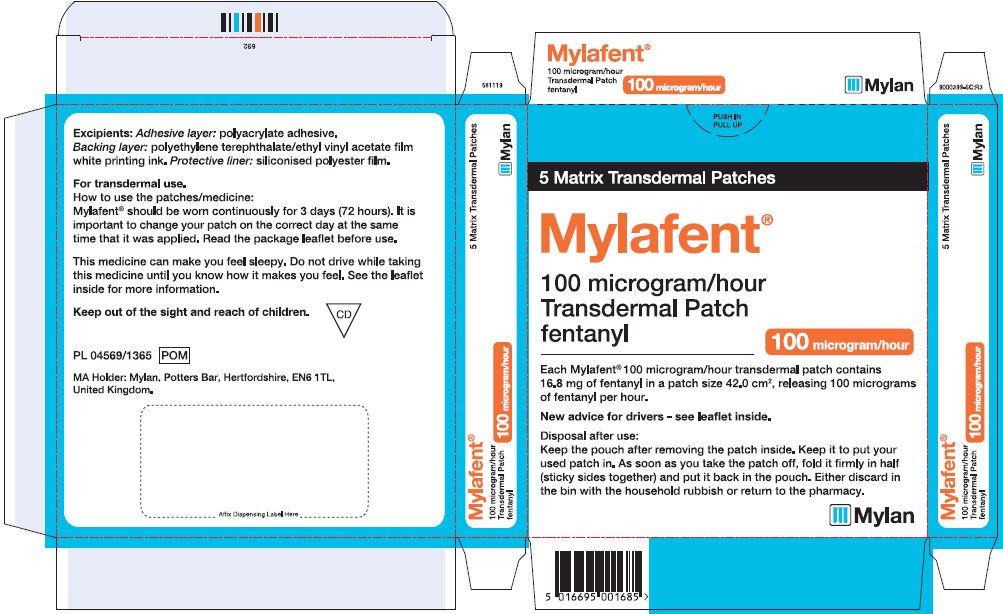
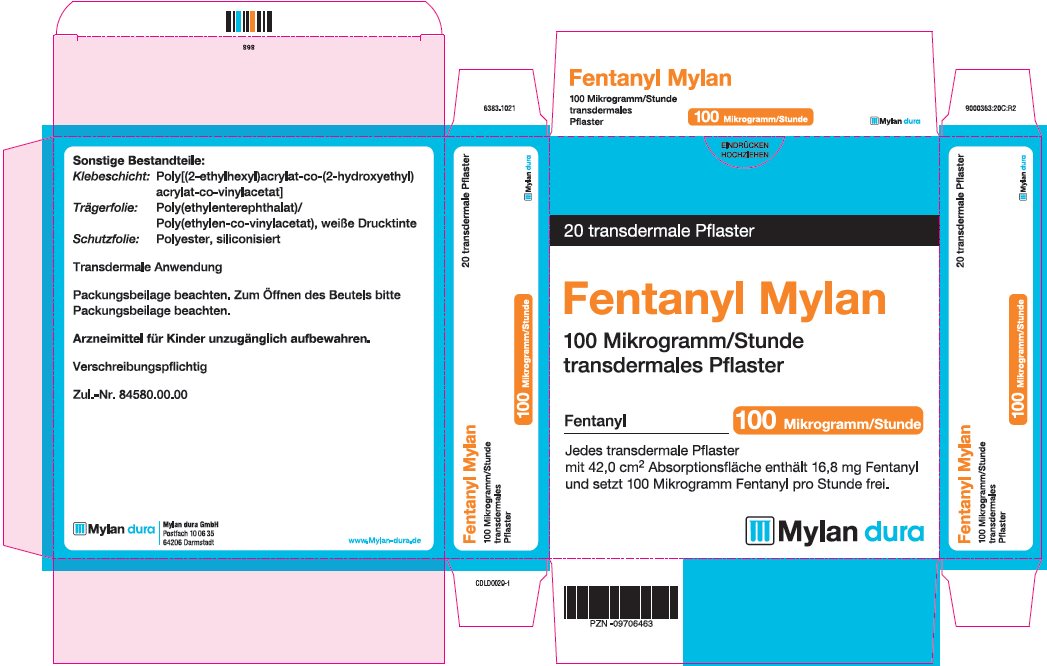
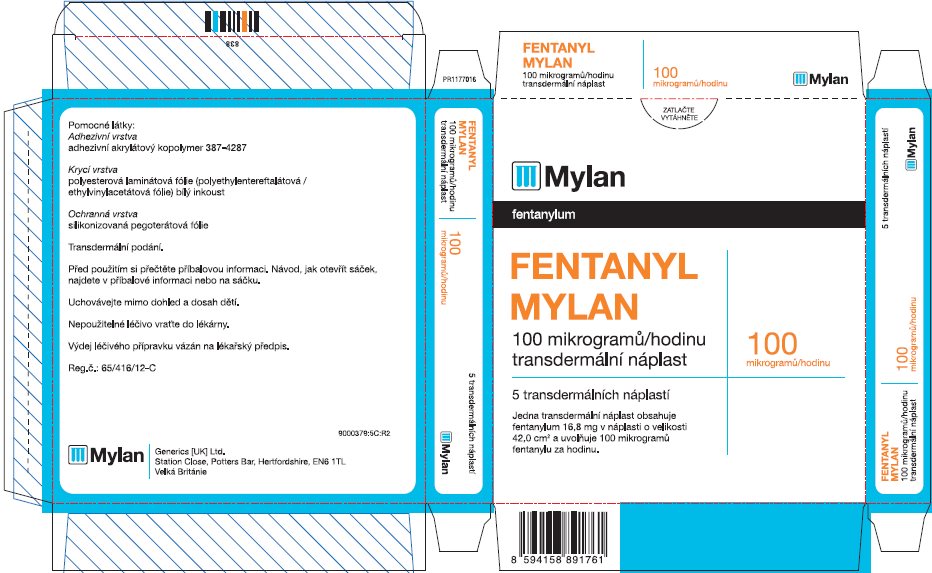
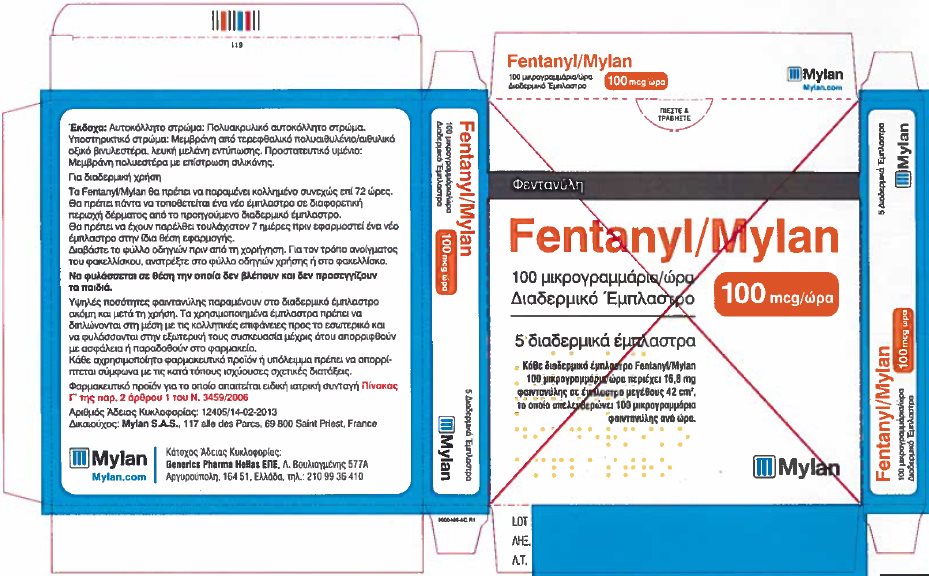
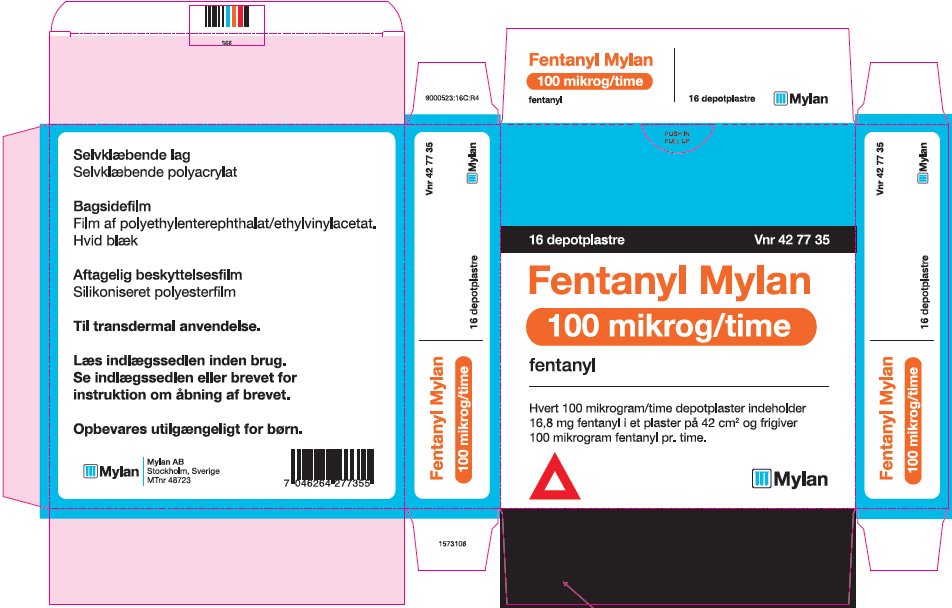
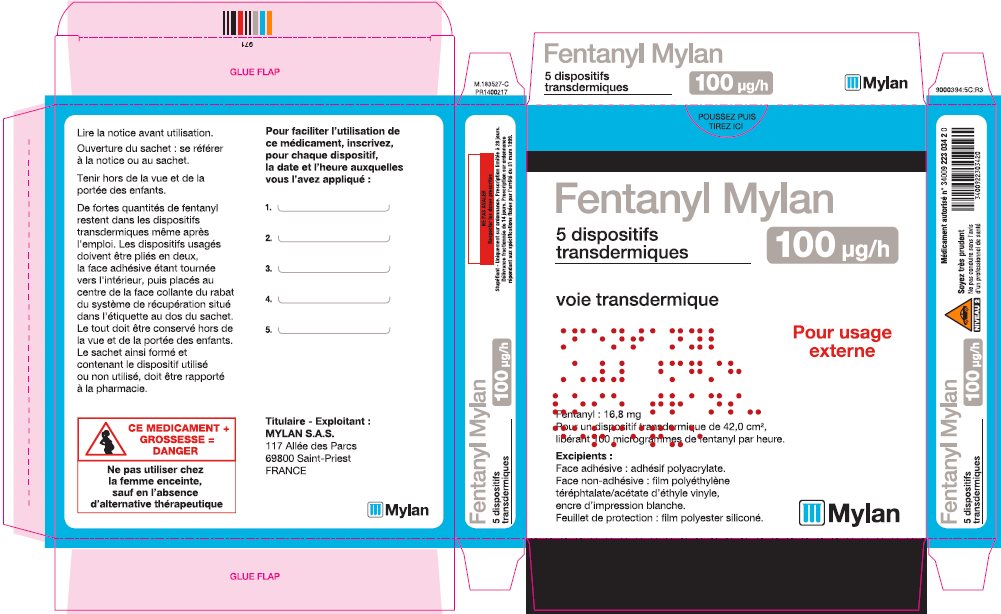
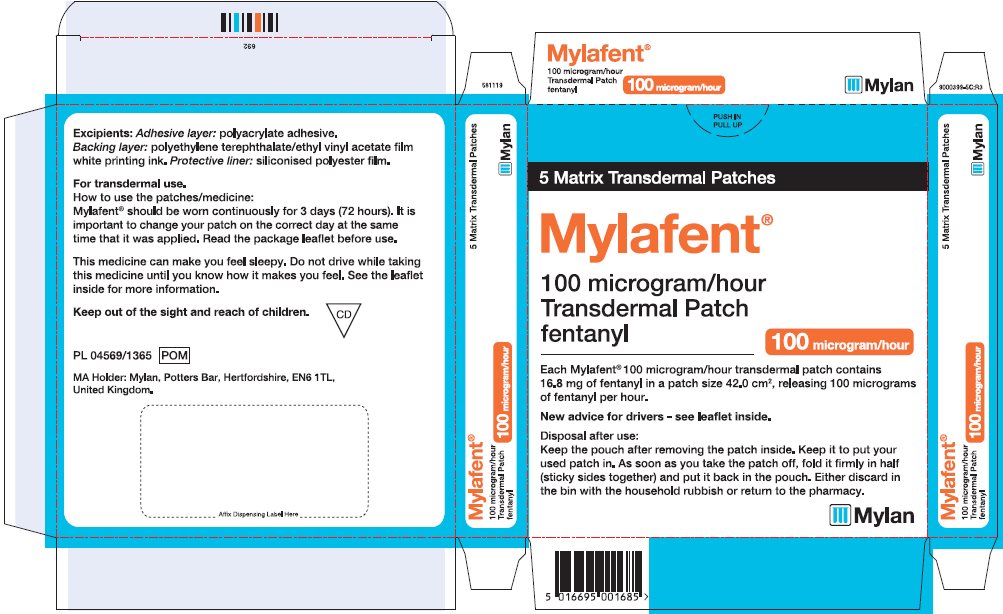
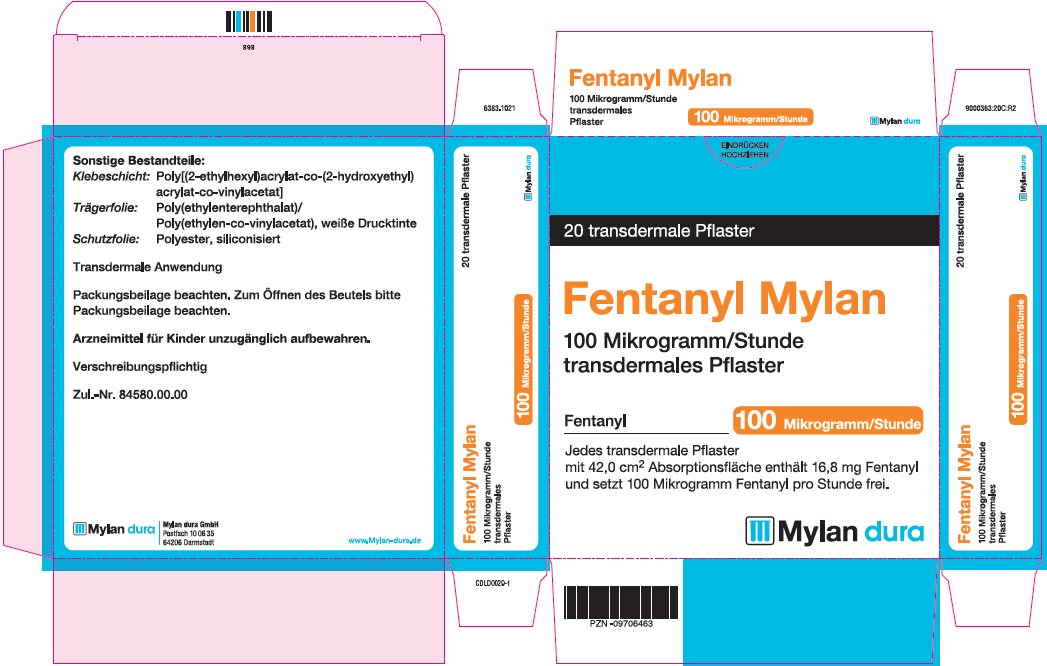
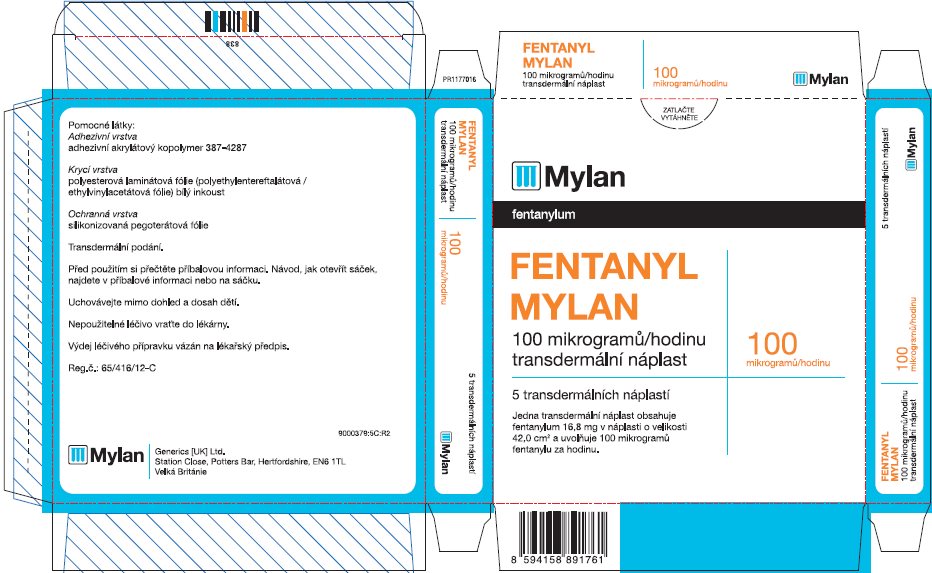
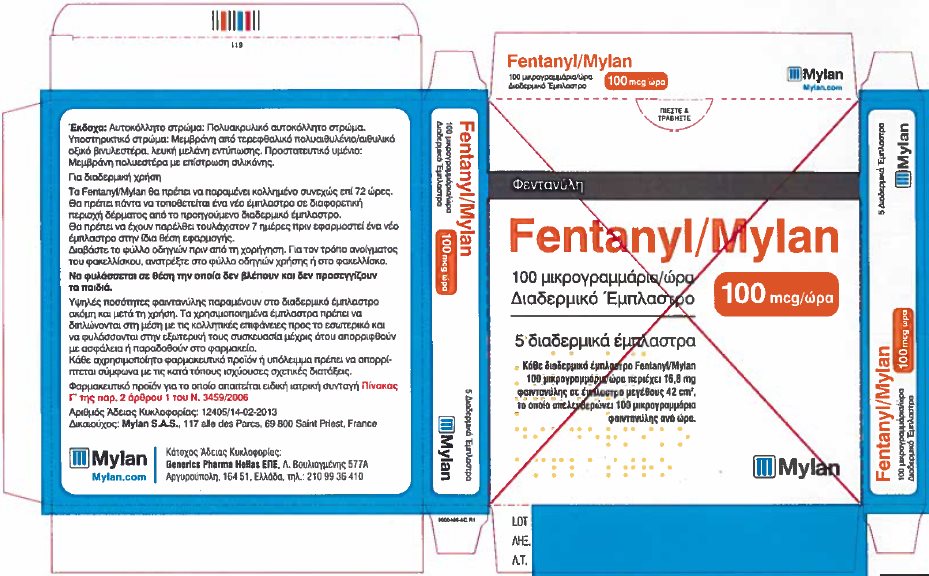
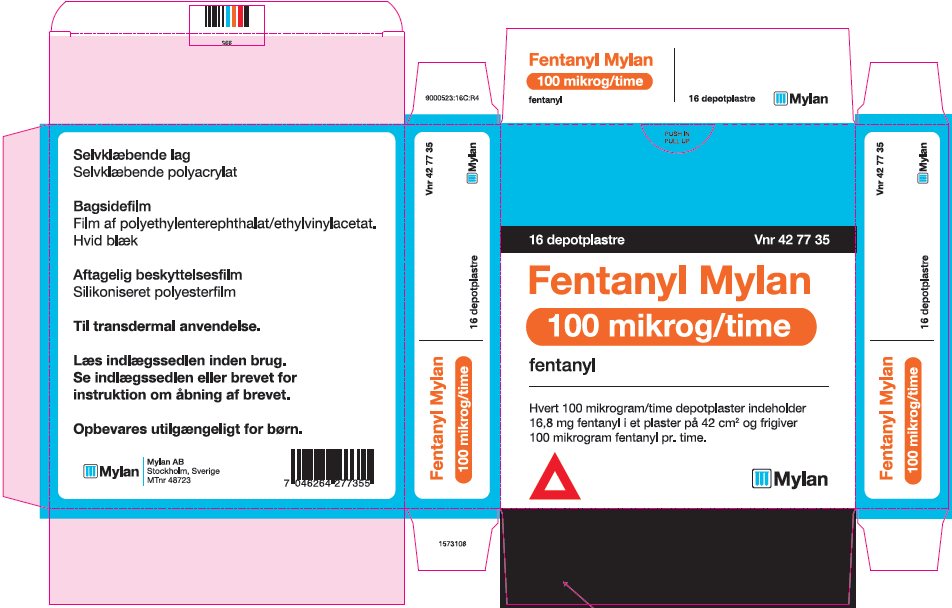
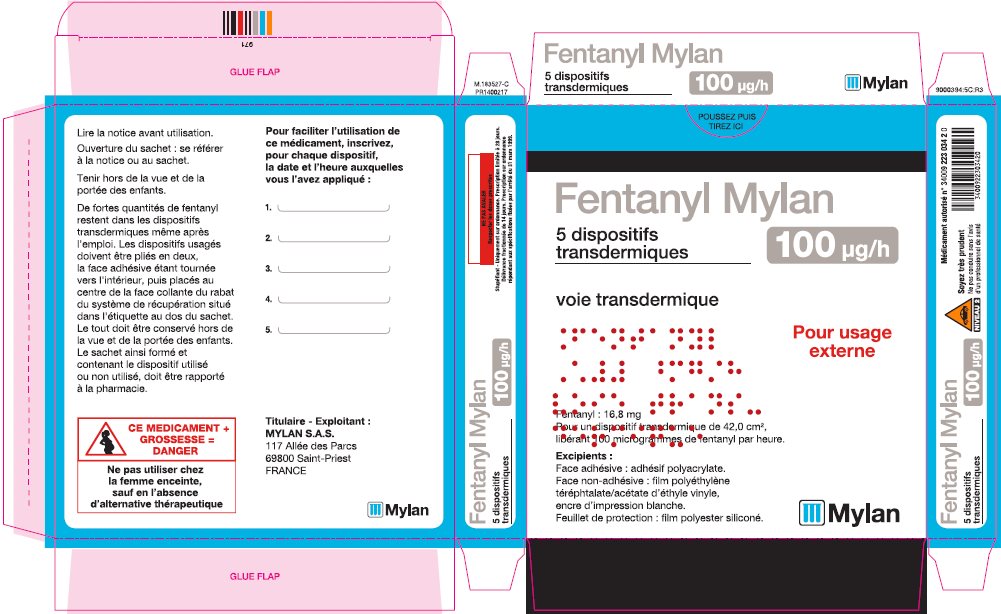
- PRINCIPAL DISPLAY PANEL – 100 microgram/hour
-
INGREDIENTS AND APPEARANCE
FENTANYL
fentanyl patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59490-4190 Route of Administration TRANSDERMAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FENTANYL (UNII: UF599785JZ) (FENTANYL - UNII:UF599785JZ) FENTANYL 12 ug in 1 h Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59490-4190-9 5 in 1 CARTON 01/01/2019 1 1 in 1 POUCH 1 72 h in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2019 FENTANYL
fentanyl patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59490-4191 Route of Administration TRANSDERMAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FENTANYL (UNII: UF599785JZ) (FENTANYL - UNII:UF599785JZ) FENTANYL 25 ug in 1 h Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59490-4191-9 5 in 1 CARTON 01/01/2019 1 1 in 1 POUCH 1 72 h in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2019 FENTANYL
fentanyl patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59490-4192 Route of Administration TRANSDERMAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FENTANYL (UNII: UF599785JZ) (FENTANYL - UNII:UF599785JZ) FENTANYL 50 ug in 1 h Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59490-4192-9 5 in 1 CARTON 01/01/2019 1 1 in 1 POUCH 1 72 h in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2019 FENTANYL
fentanyl patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59490-4193 Route of Administration TRANSDERMAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FENTANYL (UNII: UF599785JZ) (FENTANYL - UNII:UF599785JZ) FENTANYL 75 ug in 1 h Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59490-4193-9 5 in 1 CARTON 01/01/2019 1 1 in 1 POUCH 1 72 h in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2019 FENTANYL
fentanyl patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59490-4194 Route of Administration TRANSDERMAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FENTANYL (UNII: UF599785JZ) (FENTANYL - UNII:UF599785JZ) FENTANYL 100 ug in 1 h Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59490-4194-9 5 in 1 CARTON 01/01/2019 1 1 in 1 POUCH 1 72 h in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2019 Labeler - Mylan Technologies Inc. (063790265)