Label: IBUPROFEN tablet, film coated
-
NDC Code(s):
69367-394-01,
69367-394-05,
69367-395-01,
69367-395-05, view more69367-396-01, 69367-396-05
- Packager: Westminster Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use. [See WARNINGS and PRECAUTIONS].
- Ibuprofen tablets are contraindicated in the setting of coronary artery bypass graft (CABG) surgery [see CONTRAINDICATIONS and WARNINGS].
Gastrointestinal Risk
- NSAIDS cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. [See WARNINGS].
-
DESCRIPTION
Ibuprofen tablets, USP contain the active ingredient ibuprofen, which is (±) - 2 - (p - isobutylphenyl) propionic acid. Ibuprofen is a white powder with a melting point of 74-77°C and is very slightly soluble in water (<1 mg/mL) and readily soluble in organic solvents such as ethanol and acetone. The structural formula is represented below:
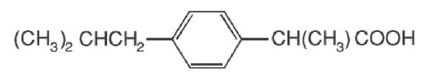
Ibuprofen, a nonsteroidal anti-inflammatory drug (NSAID), is available in 400 mg, 600 mg, and 800 mg tablets for oral administration. Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, pregelatinized starch, polyethylene glycol, soidum starch glycoate, stearic acid, talc, titanium dioxide.
-
CLINICAL PHARMACOLOGY
Ibuprofen tablets contain ibuprofen which possesses analgesic and antipyretic activities. Its mode of action, like that of other NSAIDs, is not completely understood, but may be related to prostaglandin synthetase inhibition.
In clinical studies in patients with rheumatoid arthritis and osteoarthritis, Ibuprofen tablets have been shown to be comparable to aspirin in controlling pain and inflammation and to be associated with a statistically significant reduction in the milder gastrointestinal side effects [see ADVERSE REACTIONS]. Ibuprofen may be well tolerated in some patients who have had gastrointestinal side effects with aspirin, but these patients when treated with Ibuprofen tablets should be carefully followed for signs and symptoms of gastrointestinal ulceration and bleeding. Although it is not definitely known whether ibuprofen causes less peptic ulceration than aspirin, in one study involving 885 patients with rheumatoid arthritis treated for up to one year, there were no reports of gastric ulceration with ibuprofen whereas frank ulceration was reported in 13 patients in the aspirin group (statistically significant p<.001).
Gastroscopic studies at varying doses show an increased tendency toward gastric irritation at higher doses. However, at comparable doses, gastric irritation is approximately half that seen with aspirin. Studies using 51Cr-tagged red cells indicate that fecal blood loss associated with Ibuprofen tablets in doses up to 2400 mg daily did not exceed the normal range, and was significantly less than that seen in aspirintreated patients.
In clinical studies in patients with rheumatoid arthritis, ibuprofen has been shown to be comparable to indomethacin in controlling the signs and symptoms of disease activity and to be associated with a statistically significant reduction of the milder gastrointestinal [see ADVERSE REACTIONS] and CNS side effects.
Ibuprofen may be used in combination with gold salts and/or corticosteroids.
Controlled studies have demonstrated that ibuprofen is a more effective analgesic than propoxyphene for the relief of episiotomy pain, pain following dental extraction procedures, and for the relief of the symptoms of primary dysmenorrhea.
In patients with primary dysmenorrhea, ibuprofen has been shown to reduce elevated levels of prostaglandin activity in the menstrual fluid and to reduce resting and active intrauterine pressure, as well as the frequency of uterine contractions. The probable mechanism of action is to inhibit prostaglandin synthesis rather than simply to provide analgesia.
Pharmacodynamics
In a healthy volunteer study, ibuprofen 400 mg given once daily, administered 2 hours prior to immediate-release aspirin (81 mg) for 6 days, showed an interaction with the antiplatelet activity of aspirin as measured by % serum thromboxane B2 (TxB2) inhibition at 24 hours following the day-6 aspirin dose [53%]. An interaction was still observed, but minimized, when ibuprofen 400 mg given once-daily was administered as early as 8 hours prior to the immediate-release aspirin dose [90.7%]. However, there was no interaction with the antiplatelet activity of aspirin when ibuprofen 400 mg, given once daily, was administered 2 hours after (but not concomitantly, 15 min, or 30 min after) the immediate-release aspirin dose [99.2%].
In another study, where immediate-release aspirin 81 mg was administered once daily with ibuprofen 400 mg given three times daily (1, 7, and 13 hours post-aspirin dose) for 10 consecutive days, the mean % serum thromboxane B2 (TxB2) inhibition suggested no interaction with the antiplatelet activity of aspirin [98.3%]. However, there were individual subjects with serum TxB2 inhibition below 95%, with the lowest being 90.2%.
When a similarly designed study was conducted with enteric-coated aspirin, where healthy subjects were administered enteric-coated aspirin 81 mg once daily for 6 days and ibuprofen 400 mg three times daily (2, 7 and 12 h post-aspirin dose) for 6 days, there was an interaction with the antiplatelet activity at 24 hours following the day-6 aspirin dose [67%]. [See Precautions/Drug Interactions].
Pharmacokinetics
The ibuprofen in Ibuprofen tablets is rapidly absorbed. Peak serum ibuprofen levels are generally attained one to two hours after administration. With single doses up to 800 mg, a linear relationship exists between amount of drug administered and the integrated area under the serum drug concentration vs time curve. Above 800 mg, however, the area under the curve increases less than proportional to increases in dose. There is no evidence of drug accumulation or enzyme induction.
The administration of Ibuprofen tablets either under fasting conditions or immediately before meals yields quite similar serum ibuprofen concentration-time profiles. When ibuprofen is administered immediately after a meal, there is a reduction in the rate of absorption but no appreciable decrease in the extent of absorption. The bioavailability of the drug is minimally altered by the presence of food.
A bioavailability study has shown that there was no interference with the absorption of ibuprofen when given in conjunction with an antacid containing both aluminum hydroxide and magnesium hydroxide.
Ibuprofen is rapidly metabolized and eliminated in the urine. The excretion of ibuprofen is virtually complete 24 hours after the last dose. The serum half-life is 1.8 to 2.0 hours.
Studies have shown that following ingestion of the drug, 45% to 79% of the dose was recovered in the urine within 24 hours as metabolite A (25%), (+)-2-[ p-(2hydroxymethyl-propyl) phenyl] propionic acid and metabolite B (37%), (+)-2-[ p-(2carboxypropyl)phenyl]propionic acid; the percentages of free and conjugated ibuprofen were approximately 1% and 14%, respectively.
-
INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of Ibuprofen tablets and other treatment options before deciding to use ibuprofen. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals [see WARNINGS].
Ibuprofen tablets are indicated for relief of the signs and symptoms of rheumatoid arthritis and osteoarthritis.
Ibuprofen tablets are indicated for relief of mild to moderate pain.
Ibuprofen tablets are also indicated for the treatment of primary dysmenorrhea.
Controlled clinical trials to establish the safety and effectiveness of Ibuprofen tablets in children have not been conducted.
-
CONTRAINDICATIONS
Ibuprofen tablets are contraindicated in patients with known hypersensitivity to ibuprofen.
Ibuprofen tablets should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients [see WARNINGS, Anaphylactoid Reactions, and PRECAUTIONS, Preexisting Asthma].
Ibuprofen tablets are contraindicated in the setting of coronary artery bypass graft (CABG) surgery [see WARNINGS].
-
WARNINGS
CARDIOVASCULAR EFFECTS
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction (MI) and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDs. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, Patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID-treated patients, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as Ibuprofen, increase the risk of serious gastrointestinal (GI) events [see WARNINGS].
Status Post Coronary Artery Bypass Graft (CABG) Surgery
Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke. NSAIDs are contraindicated in the setting of CABG [see CONTRAINDICATIONS].
Post-MI Patients
Observational studies conducted in the Danish National Registry have demonstrated that patients treated with NSAIDs in the post-MI period were at increased risk of reinfarction, CV-related death, and all-cause mortality beginning in the first week of treatment. In this same cohort, the incidence of death in the first year post MI was 20 per 100 person years in NSAID-treated patients compared to 12 per 100 person years in non-NSAID exposed patients. Although the absolute rate of death declined somewhat after the first year post-MI, the increased relative risk of death in NSAID users persisted over at least the next four years of follow-up.
Avoid the use of Ibuprofen tablets in patients with a recent MI unless the benefits are expected to outweigh the risk of recurrent CV thrombotic events. If Ibuprofen tablets are used in patients with a recent MI, monitor patients for signs of cardiac ischemia.
Hypertension
NSAIDs including Ibuprofen tablets, can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including Ibuprofen tablets, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Heart Failure and Edema
The Coxib and traditional NSAID Trialists' Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalizations for heart failure in COX-2 selective-treated patients and nonselective NSAID-treated patients compared to placebotreated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of Ibuprofen may blunt the CV effects of several therapeutic agents used to treat these medical conditions [e.g., diuretics, ACE inhibitors, or angiotensin receptor blockers (ARBs)] [See Drug Interactions].
Avoid the use of Ibuprofen tablets in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If Ibuprofen tablets are used in patients with severe heart failure, monitor patients for signs of worsening heart failure.
Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation
NSAIDs, including Ibuprofen tablets, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3-6 months, and in about 2-4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk. NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients treated with neither of these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population. To minimize the potential risk for an adverse GI event in patients treated with a NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain Alert for signs and symptoms of GI ulcerations and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high-risk patients, alternate therapies that do not involve NSAIDs should be considered.
Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a NSAID may cause a dose dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Advanced Renal Disease
No information is available from controlled clinical studies regarding the use of Ibuprofen tablets in patients with advanced renal disease. Therefore, treatment with Ibuprofen tablets is not recommended in these patients with advanced renal disease. If Ibuprofen tablet therapy must be initiated, close monitoring of the patients renal function is advisable.
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to Ibuprofen tablets. Ibuprofen tablets should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs [see CONTRAINDICATIONS and PRECAUTIONS, Preexisting Asthma]. Emergency help should be sought in cases where an anaphylactoid reaction occurs.
Skin Reactions
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as Ibuprofen tablets. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, reaction, lymphadenopathy, and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present.
Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though reaction is not evident. If such signs or symptoms are present, discontinue Ibuprofen tablets and evaluate the patient immediately.
NSAIDs, including Ibuprofen tablets, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin reaction or any other sign of hypersensitivity.
Fetal Toxicity
Premature Closure of Fetal Ductus Arteriosus
Avoid use of NSAIDs, including Ibuprofen tablets, in pregnant women at about 30 weeks gestation and later. NSAIDs including Ibuprofen tablets, increase the risk of premature closure of the fetal ductus arteriosus at approximately this gestational age.
Oligohydramnios/Neonatal Renal Impairment
Use of NSAIDs, including Ibuprofen tablets, at about 20 weeks gestation or later in pregnancy may cause fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation.
Oligohydramnios is often, but not always, reversible with treatment discontinuation. Complications of prolonged oligohydramnios may, for example, include limb contractures and delayed lung maturation. In some postmarketing cases of impaired neonatal renal function, invasive procedures such as exchange transfusion or dialysis were required.
If NSAID treatment is necessary between about 20 weeks and 30 weeks gestation, limit Ibuprofen tablets use to the lowest effective dose and shortest duration possible. Consider ultrasound monitoring of amniotic fluid if Ibuprofen tablets treatment extends beyond 48 hours. Discontinue Ibuprofen tablets if oligohydramnios occurs and follow up according to clinical practice [see PRECAUTIONS; Pregnancy].
-
PRECAUTIONS
General
Ibuprofen tablets cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of Ibuprofen tablets in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
Hepatic effects
Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs, including Ibuprofen tablets. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice, fulminant hepatitis, liver necrosis, and hepatic failure, some of them with fatal outcomes have been reported. A patient with symptoms and/ or signs suggesting liver dysfunction, or with abnormal liver test values, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with Ibuprofen tablets. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, reaction, etc.), Ibuprofen tablets should be discontinued.
Hematological effects
Anemia is sometimes seen in patients receiving NSAIDs, including Ibuprofen tablets. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including Ibuprofen tablets, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
In two postmarketing clinical studies the incidence of a decreased hemoglobin level was greater than previously reported. Decrease in hemoglobin of 1 gram or more was observed in 17.1% of 193 patients on 1600 mg ibuprofen daily (osteoarthritis), and in 22.8% of 189 patients taking 2400 mg of ibuprofen daily (rheumatoid arthritis). Positive stool occult blood tests and elevated serum creatinine levels were also observed in these studies.
NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving Ibuprofen tablets who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants should be carefully monitored.
Preexisting asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and NSAIDs has been reported in such aspirin-sensitive patients, Ibuprofen tablets should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.
Ophthalmological effects
Blurred and/or diminished vision, scotomata, and/or changes in color vision have been reported. If a patient develops such complaints while receiving Ibuprofen tablets, the drug should be discontinued, and the patient should have an ophthalmologic examination which includes central visual fields and color vision testing.
Aseptic Meningitis
Aseptic meningitis with fever and coma has been observed on rare occasions in patients on ibuprofen therapy. Although it is probably more likely to occur in patients with systemic lupus erythematosus and related connective tissue diseases, it has been reported in patients who do not have an underlying chronic disease. If signs or symptoms of meningitis develop in a patient on Ibuprofen tablets, the possibility of its being related to Ibuprofen tablets should be considered.
Information for Patients
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
-
CARDIOVASCULAR THROMBOTIC EVENTS
Advise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their health care provider immediately [see WARNINGS]. - Ibuprofen tablets, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative signs or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up [see WARNINGS, Gastrointestinal Effects-Risk of Ulceration, Bleeding and Perforation].
- Ibuprofen tablets, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS and TEN, which may result in hospitalization and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin Reaction and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative sign or symptoms.
Patients should be advised to stop the drug immediately if they develop any type of reaction and contact their physicians as soon as possible. - Serious Skin Reactions, including DRESS: Advise patients to stop taking Ibuprofen tablets immediately if they develop any type of reaction or fever and to contact their healthcare provider as soon as possible [see WARNINGS].
- Heart failure and Edema: Advise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur [see WARNINGS].
- Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness and "flu-like" symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
- Patients should be informed of the signs of an anaphylactoid reaction (e.g. difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help [see WARNINGS].
- In late pregnancy, as with other NSAIDs, Ibuprofen tablets should be avoided because it may cause premature closure of the ductus arteriosus.
Fetal Toxicity
Inform pregnant women to avoid use of Ibuprofen tablets and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus. If treatment with Ibuprofen tablets is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours [see WARNINGS; Fetal Toxicity, PRECAUTIONS; Pregnancy].
Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, reaction etc.), or abnormal liver tests persist or worsen, Ibuprofen tablets should be discontinued.
Drug Interactions
ACE-inhibitors
Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors.
Aspirin
Pharmacodynamic studies have demonstrated interference with the antiplatelet activity of aspirin when ibuprofen 400 mg, given three times daily, is administered with enteric-coated low-dose aspirin. The interaction exists even following a once-daily regimen of ibuprofen 400 mg, particularly when ibuprofen is dosed prior to aspirin. The interaction is alleviated if immediate-release low-dose aspirin is dosed at least 2 hours prior to a once-daily regimen of ibuprofen; however, this finding cannot be extended to enteric-coated low-dose aspirin [see Clinical Pharmacology/Pharmacodynamics].
Because there may be an increased risk of cardiovascular events due to the interference of ibuprofen with the antiplatelet effect of aspirin, for patients taking low-dose aspirin for cardio protection who require analgesics, consider use of a NSAID that does not interfere with the antiplatelet effect of aspirin, or non-NSAID analgesics, where appropriate.
When Ibuprofen tablets are administered with aspirin, its protein binding is reduced, although the clearance of free Ibuprofen tablets is not altered. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of ibuprofen and aspirin is not generally recommended because of the potential for increased adverse effects.
Diuretics
Clinical studies, as well as post marketing observations, have shown that Ibuprofen tablets can reduce the natriuretic effect-of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure [see PRECAUTIONS, Renal Effects], as well as to assure diuretic efficacy.
Lithium
Ibuprofen produced an elevation of plasma lithium levels and a reduction in renal lithium clearance in a study of eleven normal volunteers. The mean minimum lithium concentration increased 15% and the renal clearance of lithium was decreased by 19% during this period of concomitant drug administration.
This effect has been attributed to inhibition of renal prostaglandin synthesis by ibuprofen. Thus, when ibuprofen and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity. (Read circulars for lithium preparation before use of such concurrent therapy.)
Methotrexate
NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
Warfarin-type anticoagulants
Several short-term controlled studies failed to show that Ibuprofen tablets significantly affected prothrombin times or a variety of other clotting factors when administered to individuals on coumarin-type anticoagulants. However, because bleeding has been reported when Ibuprofen tablets and other NSAIDs have been administered to patients on coumarin-type anticoagulants, the physician should be cautious when administering Ibuprofen tablets to patients on anticoagulants. The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that the users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
Pregnancy
Risk Summary
Use of NSAIDs, including Ibuprofen tablets, can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. Because of these risks, limit dose and duration of Ibuprofen tablets use between about 20 and 30 weeks of gestation, and avoid Ibuprofen tablets use at about 30 weeks of gestation and later in pregnancy [see WARNINGS; Fetal Toxicity].
Premature Closure of Fetal Ductus Arteriosus
Use of NSAIDs, including Ibuprofen tablets, at about 30 weeks gestation or later in pregnancy increases the risk of premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment
Use of NSAIDs at about 20 weeks gestation or later in pregnancy has been associated with cases of fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment.
Data from observational studies regarding other potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive. Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as Ibuprofen, resulted in increased pre- and post-implantation loss. Prostaglandins also have been shown to have an important role in fetal kidney development. In published animal studies, prostaglandin synthesis inhibitors have been reported to impair kidney development when administered at clinically relevant doses.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Premature Closure of Fetal Ductus Arteriosus: Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy, because NSAIDs, including Ibuprofen tablets, can cause premature closure of the fetal ductus arteriosus [see WARNINGS; Fetal Toxicity].
Oligohydramnios/Neonatal Renal Impairment
If an NSAID is necessary at about 20 weeks gestation or later in pregnancy, limit the use to the lowest effective dose and shortest duration possible. If Ibuprofen tablets treatment extends beyond 48 hours, consider monitoring with ultrasound for oligohydramnios. If oligohydramnios occurs, discontinue Ibuprofen tablets and follow up according to clinical practice [see WARNINGS; Fetal Toxicity].
Data
Human Data
There are no adequate, well-controlled studies in pregnant women. Ibuprofen tablets should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Premature Closure of Fetal Ductus Arteriosus
Published literature reports that the use of NSAIDs at about 30 weeks of gestation and later in pregnancy may cause premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment
Published studies and postmarketing reports describe maternal NSAID use at about 20 weeks gestation or later in pregnancy associated with fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. In many cases, but not all, the decrease in amniotic fluid was transient and reversible with cessation of the drug. There have been a limited number of case reports of maternal NSAID use and neonatal renal dysfunction without oligohydramnios, some of which were irreversible. Some cases of neonatal renal dysfunction required treatment with invasive procedures, such as exchange transfusion or dialysis.
Methodological limitations of these postmarketing studies and reports include lack of a control group; limited information regarding dose, duration, and timing of drug exposure; and concomitant use of other medications. These limitations preclude establishing a reliable estimate of the risk of adverse fetal and neonatal outcomes with maternal NSAID use.
Because the published safety data on neonatal outcomes involved mostly preterm infants, the generalizability of certain reported risks to the full-term infant exposed to NSAIDs through maternal use is uncertain.
Labor and Delivery
In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of Ibuprofen tablets on labor and delivery in pregnant women are unknown.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human-milk and because of the potential for serious adverse reactions in nursing infants from Ibuprofen tablets, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
-
CARDIOVASCULAR THROMBOTIC EVENTS
-
ADVERSE REACTIONS
The most frequent type of adverse reaction occurring with Ibuprofen tablets is gastrointestinal. In controlled clinical trials the percentage of patients reporting one or more gastrointestinal complaints ranged from 4% to 16%.
In controlled studies when Ibuprofen tablets were compared to aspirin and indomethacin in equally effective doses, the overall incidence of gastrointestinal complaints was about half that seen in either the aspirin- or indomethacin-treated patients.
Adverse reactions observed during controlled clinical trials at an incidence greater than 1% are listed in the table. Those reactions listed in Column one encompass observations in approximately 3,000 patients. More than 500 of these patients were treated for periods of at least 54 weeks.
Still other reactions occurring less frequently than 1 in 100 were reported in controlled clinical trials and from marketing experience. These reactions have been divided into two categories: Column two of the table lists reactions with therapy with Ibuprofen tablets where the probability of a causal relationship exists: for the reactions in Column three, a causal relationship with Ibuprofen tablets has not been established.
Reported side effects were higher at doses of 3200 mg/day than at doses of 2400 mg or less per day in clinical trials of patients with rheumatoid arthritis. The increases in incidence were slight and still within the ranges reported in the table.
Incidence Greater than 1% (but less than 3%) Probable Causal Relationship* Precise Incidence Unknown (but less than 1%) Probable Causal Relationship† Precise Incidence Unknown (but less than 1%) Causal Relationship Unknown† - *
- Reactions occurring in 3% to 9% of patients treated with ibuprofen tablets. (Those reactions occurring in less than 3% of the patients are unmarked.)
- †
- Reactions are classified under "Probable Causal Relationship (PCR)" if there has been one positive rechallenge or if three or more cases occur which might be causally related. Reactions are classified under "Causal Relationship Unknown" if seven or more events have been reported but the criteria for PCR have not been met.
GASTROINTESTINAL
Nausea*, epigastric pain*, heartburn*, diarrhea, abdominal distress, nausea and vomiting, indigestion, constipation, abdominal cramps or Pain, fullness of GI tract (bloating or flatulence)Gastric or duodenal ulcer with bleeding and/or perforation, gastrointestinal hemorrhage, melena, gastritis, hepatitis, jaundice, abnormal liver function tests; pancreatitis CENTRAL NERVOUS SYSTEM
Dizziness*, headache, nervousnessDepression, insomnia, confusion, emotional liability, somnolence, aseptic meningitis with fever and coma (see PRECAUTIONS) Paresthesias, hallucinations, dream abnormalities, pseudotumor cerebri DERMATOLOGIC
Rash*, (including maculopapular type), pruritusVesiculobullous eruptions, urticaria, erythema multiforme, Stevens-Johnson syndrome, alopecia Toxic epidermal necrolysis, photoallergic skin reactions SPECIAL SENSES
TinnitusHearing loss, amblyopia (blurred and/or diminished vision, scotomata and/or changes in color vision) (see PRECAUTIONS) Conjunctivitis, diplopia, optic neuritis, cataracts HEMATOLOGIC Neutropenia, agranulocytosis, aplastic anemia, hemolytic anemia (sometimes Coombs positive), thrombocytopenia with or without purpura, eosinophilia, decreases in hemoglobin and hematocrit (see PRECAUTIONS) Bleeding episodes (eg epistaxis, menorrhagia) METABOLIC/ ENDOCRINE
Decreased appetiteGynecomastia, hypoglycemic reaction, acidosis CARDIOVASCULAR
Edema, fluid retention (generally responds promptly to drug discontinuation) (see PRECAUTIONS)Congestive heart failure in patients with marginal cardiac function, elevated blood pressure, palpitations Arrhythmias (sinus tachycardia, sinus bradycardia) ALLERGIC Syndrome of abdominal pain, fever, chills, nausea and vomiting; anaphylaxis; bronchospasm (see CONTRAINDICATIONS) Serum sickness, lupus erythematosus syndrome. Henoch- Schonlein vasculitis, angioedema RENAL Acute renal failure (see PRECAUTIONS), decreased creatinine clearance, poliuria, azotemia, cystitis, Hematuria Renal papillary necrosis MISCELLANEOUS Dry eyes and mouth, gingival ulcer, rhinitis -
OVERDOSAGE
Approximately 1½ hours after the reported ingestion of from 7 to 10 Ibuprofen tablets (400 mg), a 19-month old child weighing 12 kg was seen in the hospital emergency room, apneic and cyanotic, responding only to painful stimuli. This type of stimulus, however, was sufficient to induce respiration. Oxygen and parenteral fluids were given; a greenish-yellow fluid was aspirated from the stomach with no evidence to indicate the presence of ibuprofen. Two hours after ingestion the child's condition seemed stable; she still responded only to painful stimuli and continued to have periods of apnea lasting from 5 to 10 seconds. She was admitted to intensive care and sodium bicarbonate was administered as well as infusions of dextrose and normal saline.
By four hours post-ingestion she could be aroused easily, sit by herself and respond to spoken commands. Blood level of ibuprofen was 102.9 mcg/mL approximately 8½ hours after accidental ingestion. At 12 hours she appeared to be completely recovered.
In two other reported cases where children (each weighing approximately 10 kg) accidentally, acutely ingested approximately 120 mg/kg, there were no signs of acute intoxication or late sequelae. Blood level in one child 90 minutes after ingestion was 700 mcg/mL -- about 10 times the peak levels seen in absorption-excretion studies.
A 19-year old male who had taken 8,000 mg of ibuprofen over a period of a few hours complained of dizziness, and nystagmus was noted. After hospitalization, parenteral hydration and three days bed rest, he recovered with no reported sequelae.
In cases of acute overdosage, the stomach should be emptied by vomiting or lavage, though little drug will likely be recovered if more than an hour has elapsed since ingestion. Because the drug is acidic and is excreted in the urine, it is theoretically beneficial to administer alkali and induce diuresis. In addition to supportive measures, the use of oral activated charcoal may help to reduce the absorption and reabsorption of Ibuprofen tablets.
-
DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of Ibuprofen Tablets, USP and other treatment options before deciding to use Ibuprofen Tablets, USP. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals [see WARNINGS].
After observing the response to initial therapy with Ibuprofen Tablets, USP, the dose and frequency should be adjusted to suit an individual patient's needs.
Do not exceed 3200 mg total daily dose. If gastrointestinal complaints occur, administer Ibuprofen tablets with meals or milk.
Rheumatoid arthritis and osteoarthritis, including flare-ups of chronic disease
Suggested Dosage: 1200 mg-3200 mg daily (400 mg, 600 mg or 800 mg tid or qid). Individual patients may show a better response to 3200 mg daily, as compared with 2400 mg, although in well-controlled clinical trials patients on 3200 mg did not show a better mean response in terms of efficacy. Therefore, when treating patients with 3200 mg/ day, the physician should observe sufficient increased clinical benefits to offset potential increased risk.
The dose should be tailored to each patient, and may be lowered or raised depending on the severity of symptoms either at time of initiating drug therapy or as the patient responds or fails to respond.
In general, patients with rheumatoid arthritis seem to require higher doses of Ibuprofen tablets than do patients with osteoarthritis.
The smallest dose of Ibuprofen tablets that yields acceptable control should be employed. A linear blood level dose-response relationship exists with single doses up to 800 mg [See CLINICAL PHARMACOLOGY for effects of food on rate of absorption].
The availability of three tablet strengths facilitates dosage adjustment.
In chronic conditions, a therapeutic response to therapy with Ibuprofen tablets is sometimes seen in a few days to a week but most often is observed by two weeks. After a satisfactory response has been achieved, the patient's dose should be reviewed and adjusted as required.
-
HOW SUPPLIED
Ibuprofen tablets, USP are available in the following strengths, colors and sizes:
400 mg (white, oval shaped, debossed BI 4)
Bottles of 100 NDC 69367-394-01
Bottles of 500 NDC 69367-394-05600 mg (white, oval, debossed BI 6)
Bottles of 100 NDC 69367-395-01
Bottles of 500 NDC 69367-395-05800 mg (white, caplet, debossed BI 8)
Bottles of 100 NDC 69367-396-01
Bottles of 500 NDC 69367-396-05 - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 400 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 600 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 800 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69367-394 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 400 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL Size 15mm Flavor Imprint Code BI4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69367-394-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2024 2 NDC:69367-394-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202413 05/23/2024 IBUPROFEN
ibuprofen tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69367-395 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 600 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL Size 16mm Flavor Imprint Code BI6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69367-395-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2024 2 NDC:69367-395-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202413 05/23/2024 IBUPROFEN
ibuprofen tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69367-396 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 800 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL (Caplet) Size 19mm Flavor Imprint Code BI8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69367-396-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2024 2 NDC:69367-396-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202413 05/23/2024 Labeler - Westminster Pharmaceuticals, LLC (079516651)






