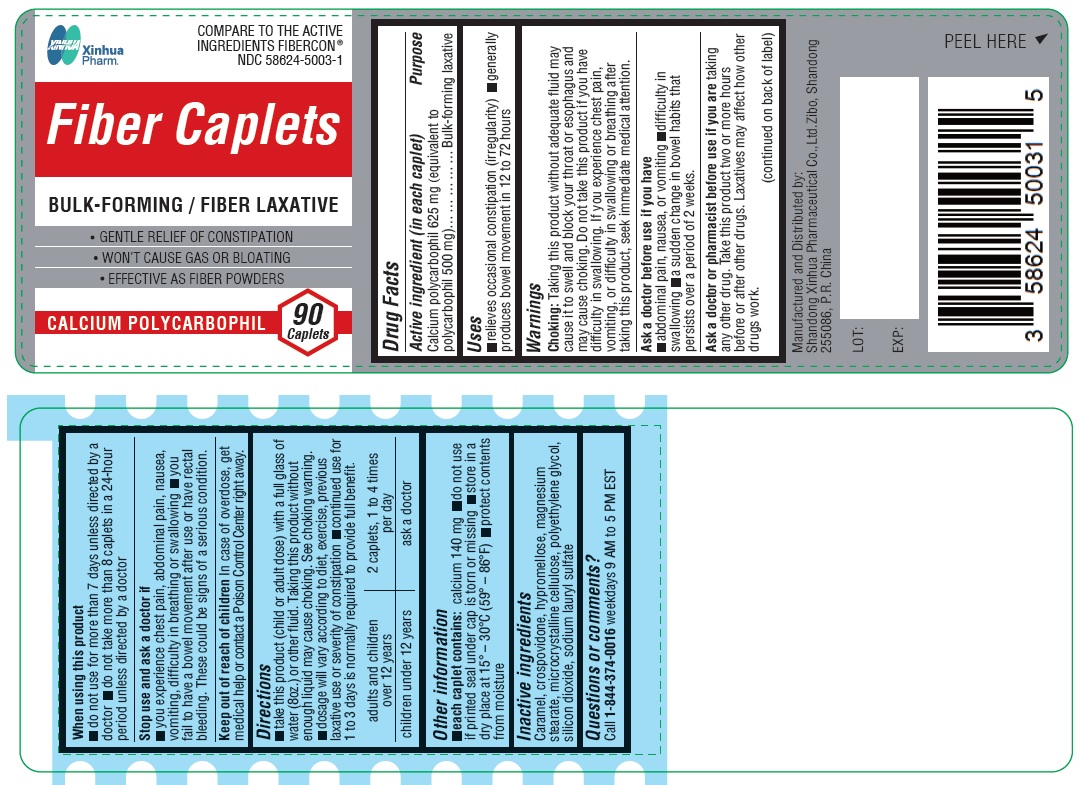
Label: CALCIUM POLYCARBOPHIL tablet, film coated
- NDC Code(s): 58624-5003-1
- Packager: SHANDONG XINHUA PHARMACEUTICAL CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
-
WARNINGS
Choking: Taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking. Do not take this product if you have difficulty in swallowing. If you experience chest pain, vomiting, or difficulty in swallowing or breathing after taking this product, seek immediate medical attention.
Ask a doctor before use if you have
abdominal pain, nausea, or vomiting
difficulty in swallowing
a sudden change in bowel habits that persists over a period of 2 weeks
Ask a doctor or pharmacist before use if you are
taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
When using this product
do not use for more than 7 days unless directed by a doctor
do not take more than 8 caplets in a 24-hour period unless directed by a doctor
-
Directions
take this product (child or adult dose) with a full glass of water (8oz.) or other fluid. Taking this product without enough liquid my cause choking. See choking warning.
dosage will vary according to diet, exercise, previous laxative use or severity of constipation
continued use for 1 to 3 days is normally required to provide full benefitadults and children over 12 years 2 caplets, 1 to 4 times per day children under 12 years ask a doctor - Other information
- INACTIVE INGREDIENT
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CALCIUM POLYCARBOPHIL
calcium polycarbophil tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58624-5003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM POLYCARBOPHIL (UNII: 8F049NKY49) (POLYCARBOPHIL - UNII:W25LM17A4W) CALCIUM POLYCARBOPHIL 625 mg Inactive Ingredients Ingredient Name Strength CARAMEL (UNII: T9D99G2B1R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CROSPOVIDONE (UNII: 2S7830E561) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color white (off white) Score 2 pieces Shape OVAL Size 19mm Flavor Imprint Code XH;C1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58624-5003-1 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/12/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 04/12/2023 Labeler - SHANDONG XINHUA PHARMACEUTICAL CO., LTD. (653915728) Registrant - SHANDONG XINHUA PHARMACEUTICAL CO., LTD. (554507599) Establishment Name Address ID/FEI Business Operations Shandong Xinhua Pharmaceutical Co., Ltd. 554507599 manufacture(58624-5003)