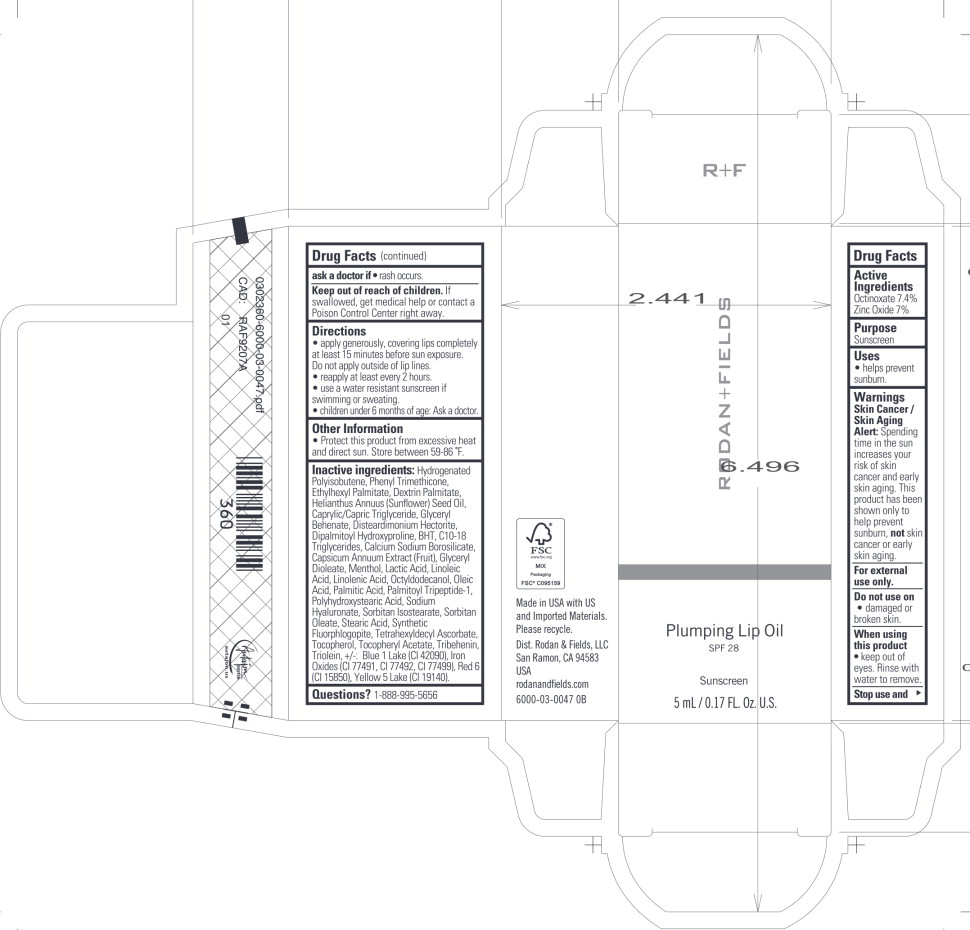
Label: RODAN AND FIELDS PLUMPING LIP SPF 28 SUNSCREEN- octinoxate, zinc oxide oil
- NDC Code(s): 14222-3000-1, 14222-3000-2, 14222-3000-3, 14222-3000-4
- Packager: Rodan & Fields
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings Skin Cancer/ Skin Aging Alert:
- Directions
- Other Information
-
Inactive ingredients:
Hydrogenated Polyisobutene, Phenyl Trimethicone, Ethylhexyl Palmitate, Dextrin Palmitate, Helianthus Annuus (Sunflower) Seed Oil, Caprylic/Capric Triglyceride, Glyceryl Behenate, Disteardimonium Hectorite, Dipalmitoyl Hydroxyproline, BHT, C10-18 Triglycerides, Calcium Sodium Borosilicate, Capsicum Annuum Extract (Fruit), Glyceryl Dioleate, Menthol, Lactic Acid, Linoleic Acid, Linolenic Acid, Octyldodecanol, Oleic Acid, Palmitic Acid, Palmitoyl Tripeptide-1, Polyhydroxystearic Acid, Sodium Hyaluronate, Sorbitan Isostearate, Sorbitan Oleate, Stearic Acid, Synthetic Fluorphlogopite, Tetrahexyldecyl Ascorbate, Tocopherol, Tocopheryl Acetate, Tribehenin, Triolein,+/-: Blue 1 Lake (Cl 42090), Iron Oxides (Cl 77491, Cl 77492, Cl 77499), Red 6 (Cl 15850), Yellow 5 Lake (Cl 19140).
- Questions?
- Principal Display Panel – 5 mL Carton Label
-
INGREDIENTS AND APPEARANCE
RODAN AND FIELDS PLUMPING LIP SPF 28 SUNSCREEN
octinoxate, zinc oxide oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-3000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 7.4 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 7 g in 100 mL Inactive Ingredients Ingredient Name Strength Hydrogenated Polyisobutene (1300 MW) (UNII: 7D1YQ9Y5EZ) Phenyl Trimethicone (UNII: DR0K5NOJ4R) Ethylhexyl Palmitate (UNII: 2865993309) Dextrin Palmitate (Corn; 20000 MW) (UNII: 89B2BSF9I3) Sunflower Oil (UNII: 3W1JG795YI) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Glyceryl Monobehenate (UNII: A626UU0W2A) Disteardimonium Hectorite (UNII: X687XDK09L) Dipalmitoyl Hydroxyproline (UNII: E6AHA53N1H) Butylated Hydroxytoluene (UNII: 1P9D0Z171K) C10-18 Triglycerides (UNII: 43AGM4PHPI) Calcium Sodium Borosilicate (UNII: 4MM76N4WMY) Glyceryl Dioleate (UNII: Z3MP1W91CW) Menthol (UNII: L7T10EIP3A) Lactic Acid (UNII: 33X04XA5AT) Linoleic Acid (UNII: 9KJL21T0QJ) Linolenic Acid (UNII: 0RBV727H71) Octyldodecanol (UNII: 461N1O614Y) Oleic Acid (UNII: 2UMI9U37CP) Palmitic Acid (UNII: 2V16EO95H1) Palmitoyl Tripeptide-1 (UNII: RV743D216M) Hyaluronate Sodium (UNII: YSE9PPT4TH) Sorbitan Isostearate (UNII: 01S2G2C1E4) Sorbitan Monooleate (UNII: 06XEA2VD56) Stearic Acid (UNII: 4ELV7Z65AP) Magnesium Potassium Aluminosilicate Fluoride (UNII: YK3DC63Y5M) Tetrahexyldecyl Ascorbate (UNII: 9LBV3F07AZ) Tocopherol (UNII: R0ZB2556P8) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Tribehenin (UNII: 8OC9U7TQZ0) Glyceryl Trioleate (UNII: O05EC62663) FD&C Blue No. 1 Aluminum Lake (UNII: J9EQA3S2JM) Ferric Oxide Red (UNII: 1K09F3G675) D&C Red No. 6 (UNII: 481744AI4O) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Polyhydroxystearic Acid (2300 MW) (UNII: YXH47AOU0F) Capsicum (UNII: 00UK7646FG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-3000-1 1 in 1 BOX 05/16/2024 1 5 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:14222-3000-2 1 in 1 BOX 05/16/2024 2 5 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:14222-3000-3 1 in 1 BOX 05/16/2024 3 5 mL in 1 TUBE; Type 0: Not a Combination Product 4 NDC:14222-3000-4 1 in 1 BOX 05/16/2024 4 5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/16/2024 Labeler - Rodan & Fields (051659584)