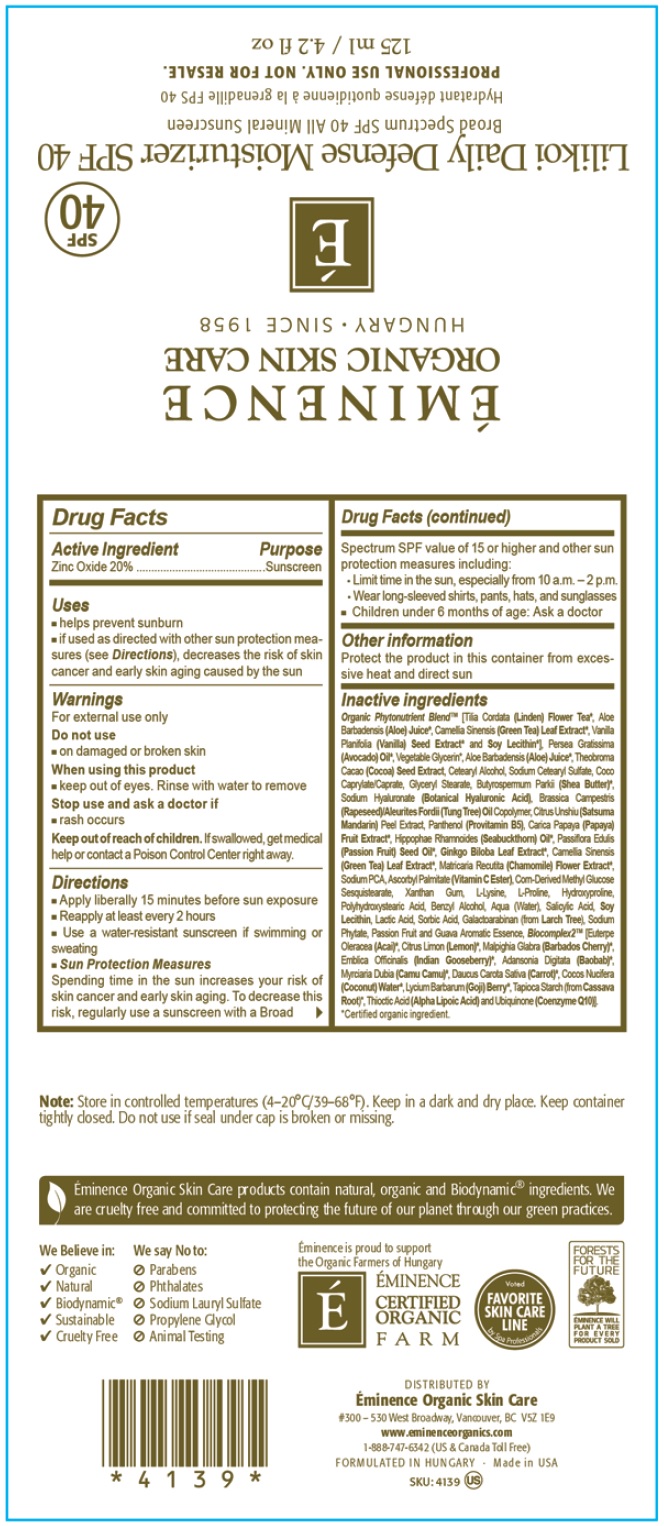
Label: EMINENCE LILIKOI DAILY DEFENSE MOISTURIZER SPF 40 SUNSCREEN- zinc oxide cream
- NDC Code(s): 15751-3501-1, 15751-3501-2, 15751-3501-3
- Packager: Eminence Organic Skin Care
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water-resistant sunscreen if swimming or sweating
- Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m
- Wear long sleeved shirts, pants, hats and sunglasses
- Children under 6 months of age: Ask a doctor
- Other information
-
Inactive Ingredients
Organic Phytonutrient Blen [Tila Cordata (Linden) Flower Tea*, Aloe Barbadensis (Aloe) Juice, Camellia Sinensis (Green Tea) Leaf Extract*, Vanilla Planifolia (Vanilla) seed Extract* and Soy Lecithin*), Persea Gratissima (Avocado) Oil*, Vegetable Glycerin*, ALoe Barbadensis (Aloe) Juice*, Theobroma Cacao (Cocoa) Seed Extract, Cetearyl Alcohol, Sodium Cetearyl Sulfate, Coco Caprylate/Caprate, Glyceryl Stearate, Butyrospermum Parkii (Shea Butter)*, Sodium Hyaluronate (Botanical Hyaluronic Acid), Brassica Campestris (Rapeseed)/Aleurites Fordii (Tung Tree) Oil Copolymer, Citrus Unshiu (Satsuma Mandarin) Peel Extract, Panthenol (Provitamin B5), Carica Papaya (Papaya) Fruit Extract, Hippophae Rhamnoides (Seabuckthorn) Oil*, Passiflora Edulis (Passion Fruit) Seed Oil*, Ginkgo Biloba Leaf Extract, Camellis Sinensis (Green Tea) Leaf Extract, Matricaria Recutita (Chamomile) Flower Extract*, Sodium PCA, Ascorbyl Palmitate (Vitamin C Ester), Corn-Derived Methyl Glucose Sesquistearate, Xanthan Gum, L-Lysine, L-Proline, Hydroxyproline, Polyhydroxystearic Acid, Benzyl Alcohol, Aqua (Water), Salicylic Acid, Soy Lecithin, Lactic Acid, Sorbic Acid, Galactoarabinan (from Larch Tree), Sodium Phytate, Passion Fruit and Guava Aromatic Essence, Blocomplex2 [Euterpe Oleracea (Acai)*, Citrus Limon (Lemon)*, Malpighia Glabra (Barbados Cherry)*, Emblica Officinalis (Indian Gooseberry)*, Adansonia Digitata (Baobab)*, Myrciaria Dubia (Camu Camu)*, Daucus Carota Daucus Carota Sativa (Carrot)*, Cocos Nucifera (Coconut) Water*, Lycium Barbarum (Goji) Berry*, Tapioca Starch (from Cassava Root)*, Thioctic Acid (Alpha Lipoic Acid) and Ubiquinone (Coenzyme Q10)].
Certified organic ingredient
- Note
- Package Labeling:60ml
- Package Labeling:125ml
- Package Labeling: 3ml
-
INGREDIENTS AND APPEARANCE
EMINENCE LILIKOI DAILY DEFENSE MOISTURIZER SPF 40 SUNSCREEN
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:15751-3501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) VANILLA BEAN (UNII: Q74T35078H) AVOCADO OIL (UNII: 6VNO72PFC1) GLYCERIN (UNII: PDC6A3C0OX) COCOA (UNII: D9108TZ9KG) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SODIUM CETOSTEARYL SULFATE (UNII: 7ZBS06BH4B) COCOYL CAPRYLOCAPRATE (UNII: 8D9H4QU99H) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SHEA BUTTER (UNII: K49155WL9Y) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) TANGERINE PEEL (UNII: JU3D414057) PANTHENOL (UNII: WV9CM0O67Z) PAPAYA (UNII: KU94FIY6JB) PASSIFLORA EDULIS SEED OIL (UNII: F3VOA31UHQ) GINKGO (UNII: 19FUJ2C58T) CHAMOMILE (UNII: FGL3685T2X) ASCORBYL PALMITATE (UNII: QN83US2B0N) XANTHAN GUM (UNII: TTV12P4NEE) LYSINE (UNII: K3Z4F929H6) PROLINE (UNII: 9DLQ4CIU6V) HYDROXYPROLINE (UNII: RMB44WO89X) BENZYL ALCOHOL (UNII: LKG8494WBH) WATER (UNII: 059QF0KO0R) SALICYLIC ACID (UNII: O414PZ4LPZ) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) LACTIC ACID (UNII: 33X04XA5AT) SORBIC ACID (UNII: X045WJ989B) GALACTOARABINAN (UNII: SL4SX1O487) HEXASODIUM PHYTATE (UNII: ZBX50UG81V) LEMON (UNII: 24RS0A988O) CARROT (UNII: L56Z1JK48B) COCONUT WATER (UNII: 267F5Y81NT) STARCH, TAPIOCA (UNII: 24SC3U704I) THIOCTIC ACID (UNII: 73Y7P0K73Y) UBIDECARENONE (UNII: EJ27X76M46) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15751-3501-1 1 in 1 BOX 01/01/2021 1 60 mL in 1 JAR; Type 0: Not a Combination Product 2 NDC:15751-3501-2 1 in 1 BOX 01/01/2021 2 125 mL in 1 JAR; Type 0: Not a Combination Product 3 NDC:15751-3501-3 3 mL in 1 PACKET; Type 0: Not a Combination Product 01/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2021 Labeler - Eminence Organic Skin Care (205753317)