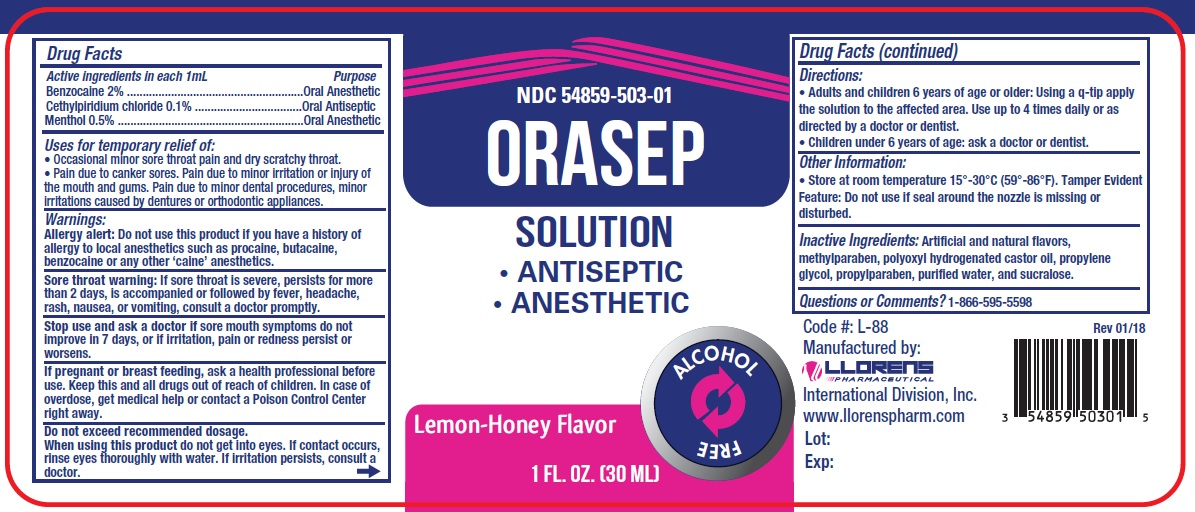
Label: ORASEP- benzocaine, menthol, cetylpyridinium chloride liquid
- NDC Code(s): 54859-503-01
- Packager: Llorens Pharmaceutical International Division
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 23, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings:
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other 'caine' anesthetics.
Sore throat warning: If sore throat is sever, persists for more than2 days, is accompanied or followed by fever, headache, rash, nausea, or vomitings, consult a doctor promptly.
Stop use and ask a doctor if sore mouth symptoms do not improve in 7 days, or if irritation, pain or redness persist or worsens.
Do not exceed recommended dosage.
When using this product do not get into eyes. If contact occurs, rinse eyes thoroughly with water. If irritation persists, consult a doctor.
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ORASEP
benzocaine, menthol, cetylpyridinium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54859-503 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 2 mg in 100 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.5 mg in 100 mL CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.1 mg in 100 mL Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54859-503-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 04/01/2010 Labeler - Llorens Pharmaceutical International Division (037342305)