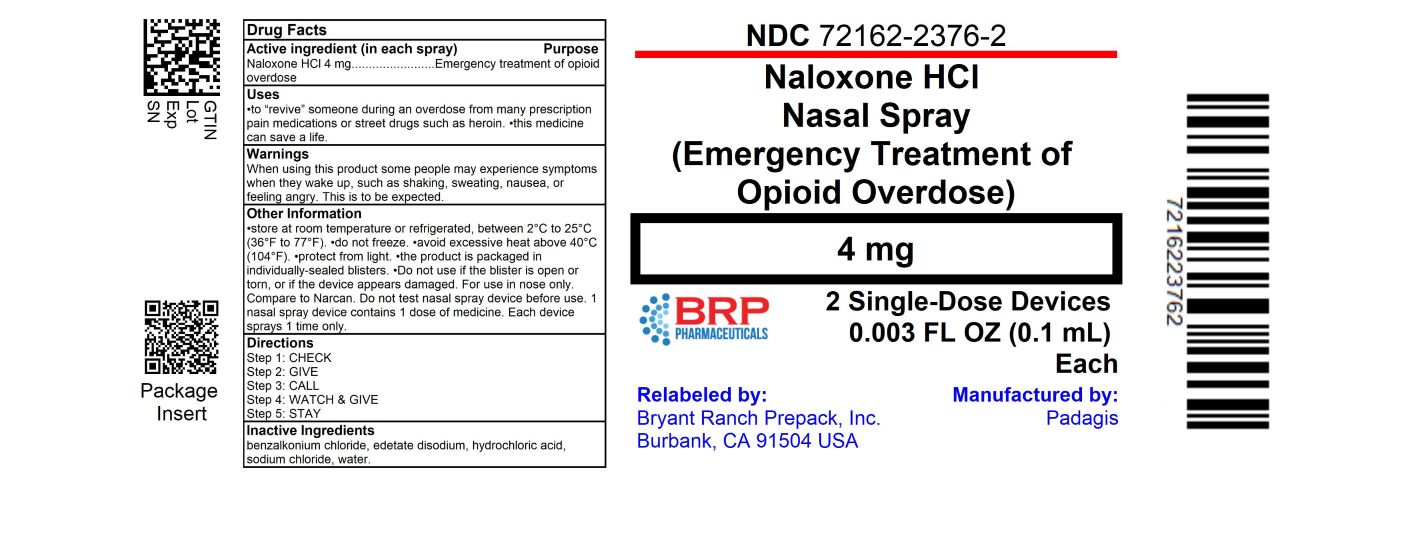
Label: NALOXONE HYDROCHLORIDE spray
- NDC Code(s): 72162-2376-2, 72162-2376-4
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 45802-578
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each spray)
- Purpose
- Uses
-
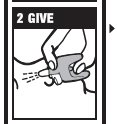
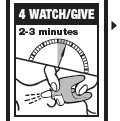
Directions
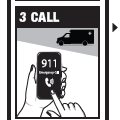
Emergency Treatment of Opioid Overdose
For opioid emergencies, call 911. For questions on Naloxone HCl Nasal Spray 4 mg, call Padagis® at 1-866-634-9120 or go to www.padagis.com.
64Q00 RT QS2
- Warning
- Other information
- Inactive Ingredients
- Questions?
- HOW SUPPLIED
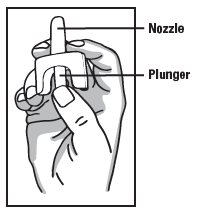
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NALOXONE HYDROCHLORIDE
naloxone hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72162-2376(NDC:45802-578) Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 4 mg in 0.1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72162-2376-2 2 in 1 CARTON 10/03/2024 1 NDC:72162-2376-4 0.1 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211951 07/30/2023 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(72162-2376) , RELABEL(72162-2376)