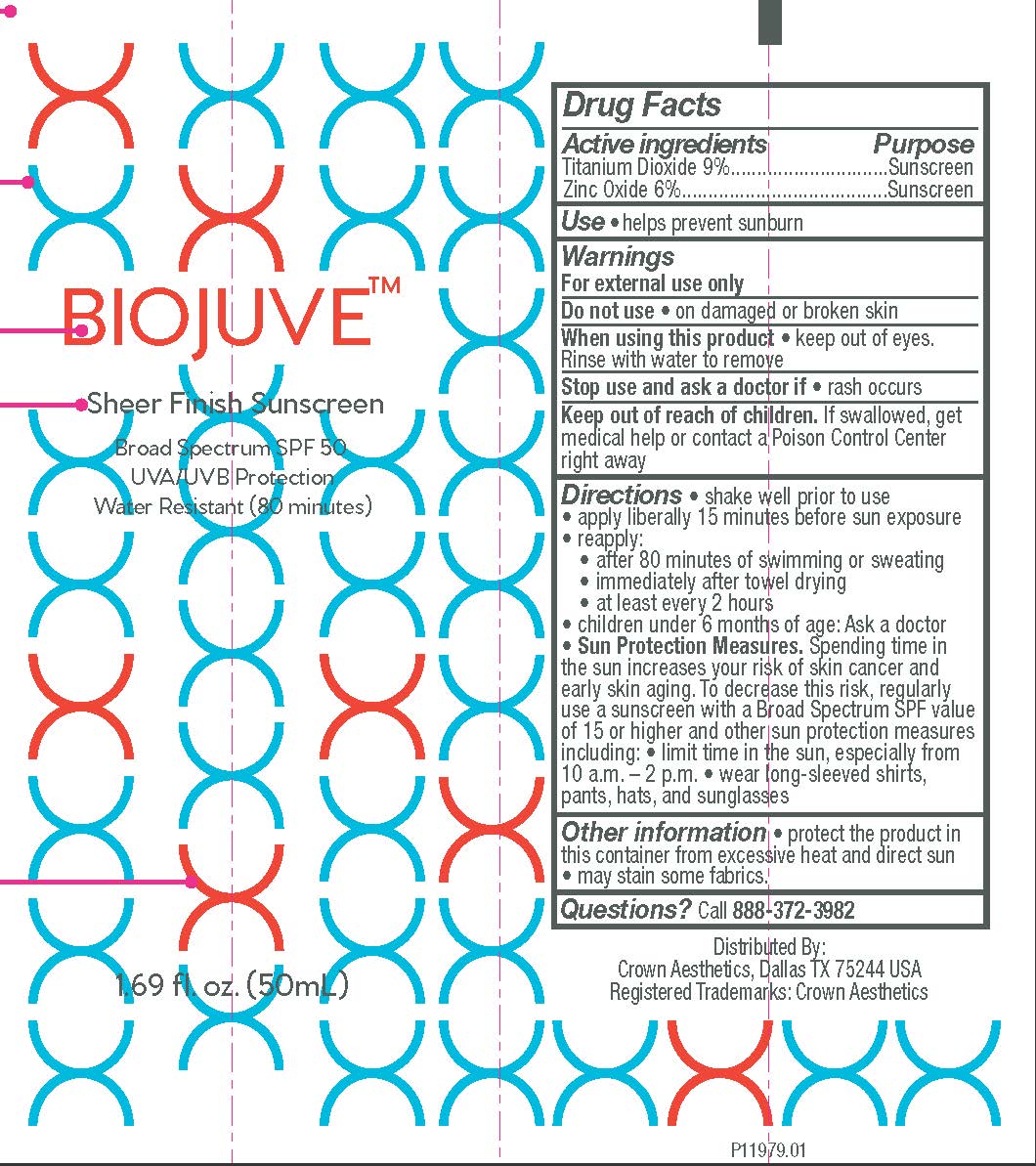
Label: BIOJUVE MICROBIOME-SAFE SHEER FINISH SPF 50 SUNSCREEN- titanium dioxide and zinc oxide lotion
- NDC Code(s): 0316-0300-50
- Packager: Crown Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- shake well prior to use
- apply liberally 15 minutes before sun exposure
- reapply:
- after 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun especially from 10 a.m.- 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Other Information
-
Inactive Ingredients
Alanine, Aloe Barbadensis Leaf Juice, Alumina, Aluminum Stearate, Arginine, Aspartic Acid, Butyrospermum Parkii (Shea) Butter, C12-15 Alkyl Benzoate, Caprylic/Capric Triglyceride, Carica Papaya (Papaya) Fruit Extract, Citric Acid, Crithmum Maritimum Extract, Cucumis Sativus (Cucumber) Fruit Extract, Diisopropyl Sebacate, Dimethoxydiphenylsilane/ Triethoxycaprylylsilane Crosspolymer, Ethylhexylglycerin, Fucus Vesiculosus (Bladderwrack) Extract, Ginkgo Biloba (Ginkgo) Leaf Extract, Glycerin, Glycine, Glycine Soja (Soybean) Oil, Hamamelis Virginiana (Witch Hazel) Leaf Extract, Histidine, Hydrogenated Vegetable Oil, Isoleucine, Isostearyl Alcohol, Isostearyl Isostearate, Oryza Sativa (Rice) Extract, Oryza Sativa (Rice) Germ Extract, PCA, Phenoxyethanol, Phenylalanine, Polyglyceryl-3 Diisostearate, Polyglyceryl-3 Polyricinoleate, Polyhydroxystearic Acid, Potassium Sorbate, Proline, Purified Water, Rosa Canina (Rosehip) Fruit Oil, Sclerocarya Birrea (Marula) Seed Oil, Serine, Sodium Benzoate, Sodium Chloride, Sodium Hyaluronate, Sodium Lactate, Sodium PCA, Sorbitan Isostearate, Squalane, Threonine, Tocopheryl Acetate (Vitamin E), Valine, and Xanthan Gum
- Questions ?
- BIOJUVE Sheer Finish SPF 50 Sunscreen - 1.69 fl oz Tube
- BIOJUVE Sheer Finish SPF 50 Sunscreen - 1.69 fl oz carton
-
INGREDIENTS AND APPEARANCE
BIOJUVE MICROBIOME-SAFE SHEER FINISH SPF 50 SUNSCREEN
titanium dioxide and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0316-0300 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 66.6 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 99.9 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM LACTATE (UNII: TU7HW0W0QT) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) THREONINE (UNII: 2ZD004190S) VALINE (UNII: HG18B9YRS7) XANTHAN GUM (UNII: TTV12P4NEE) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) WATER (UNII: 059QF0KO0R) SERINE (UNII: 452VLY9402) SODIUM BENZOATE (UNII: OJ245FE5EU) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) ISOSTEARYL ISOSTEARATE (UNII: IV0Z586Z4Y) FUCUS VESICULOSUS (UNII: 535G2ABX9M) GINKGO (UNII: 19FUJ2C58T) GLYCERIN (UNII: PDC6A3C0OX) GLYCINE (UNII: TE7660XO1C) HISTIDINE (UNII: 4QD397987E) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) HYDROGENATED RAPESEED OIL (UNII: K168T6Y0YU) SOYBEAN OIL (UNII: 241ATL177A) RICE GERM (UNII: 7N2B70SFEZ) PIDOLIC ACID (UNII: SZB83O1W42) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) ASPARTIC ACID (UNII: 30KYC7MIAI) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SHEA BUTTER (UNII: K49155WL9Y) PAPAYA (UNII: KU94FIY6JB) CRITHMUM MARITIMUM (UNII: J7IHY79BKY) ALOE VERA LEAF (UNII: ZY81Z83H0X) CUCUMBER (UNII: YY7C30VXJT) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYLALANINE (UNII: 47E5O17Y3R) ISOLEUCINE (UNII: 04Y7590D77) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SQUALANE (UNII: GW89575KF9) SCLEROCARYA BIRREA SEED OIL (UNII: WDO4TLS35F) ROSA CANINA FRUIT OIL (UNII: CR7307M3QZ) PROLINE (UNII: 9DLQ4CIU6V) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) HAMAMELIS VIRGINIANA LEAF (UNII: T07U1161SV) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DIMETHOXYDIPHENYLSILANE (UNII: 02QB6788GC) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) HYDROGENATED PALM OIL (UNII: 257THB963H) ALANINE (UNII: OF5P57N2ZX) ARGININE (UNII: 94ZLA3W45F) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0316-0300-50 1 in 1 CARTON 10/25/2022 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/25/2022 Labeler - Crown Laboratories (079035945) Establishment Name Address ID/FEI Business Operations Crown Laboratories 079035945 manufacture(0316-0300)