Label: BREVITAL SODIUM- methohexital sodium injection, powder, lyophilized, for solution
- NDC Code(s): 42023-105-01
- Packager: Endo USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: New Drug Application
Drug Label Information
Updated August 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING
BREVITAL should be used only in hospital or ambulatory care settings that provide for continuous monitoring of respiratory (e.g. pulse oximetry) and cardiac function. Immediate availability of resuscitative drugs and age- and size-appropriate equipment for bag/valve/mask ventilation and intubation and personnel trained in their use and skilled in airway management should be assured. For deeply sedated patients, a designated individual other than the practitioner performing the procedure should be present to continuously monitor the patient. (See WARNINGS.)
-
DESCRIPTION
BREVITAL® (methohexital sodium, USP) for injection, is 2,4,6 (1H, 3H, 5H)-Pyrimidinetrione, 1-methyl-5-(1-methyl-2-pentynyl)-5-(2-propenyl)-, (±)-, monosodium salt and has the empirical formula C14H17N2NaO3. Its molecular weight is 284.29.
The structural formula is as follows:
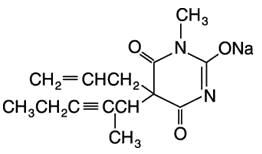
Methohexital sodium is a rapid, ultrashort-acting barbiturate anesthetic. Methohexital sodium for injection is a freeze-dried, sterile, nonpyrogenic mixture of methohexital sodium with 6% anhydrous sodium carbonate added as a buffer. It contains not less than 90% and not more than 110% of the labeled amount of methohexital sodium. It occurs as a white, freeze-dried plug that is freely soluble in water.
This product is oxygen sensitive. The pH of the 1% solution is between 10 and 11; the pH of the 0.2% solution in 5% dextrose is between 9.5 and 10.5.
Methohexital sodium may be administered by direct intravenous injection or continuous intravenous drip, intramuscular or rectal routes (see PRECAUTIONS/Pediatric Use). Reconstituting instructions vary depending on the route of administration (see DOSAGE AND ADMINISTRATION).
-
CLINICAL PHARMACOLOGY
Compared with thiamylal and thiopental, methohexital is at least twice as potent on a weight basis, and its duration of action is only about half as long. Although the metabolic fate of methohexital in the body is not clear, the drug does not appear to concentrate in fat depots to the extent that other barbiturate anesthetics do. Thus, cumulative effects are fewer and recovery is more rapid with methohexital than with thiobarbiturates. In experimental animals, the drug cannot be detected in the blood 24 hours after administration.
Methohexital differs chemically from the established barbiturate anesthetics in that it contains no sulfur. Little analgesia is conferred by barbiturates; their use in the presence of pain may result in excitation.
Intravenous administration of methohexital results in rapid uptake by the brain (within 30 seconds) and rapid induction of sleep.
Following intramuscular administration to pediatric patients, the onset of sleep occurs in 2 to 10 minutes. A plasma concentration of 3 mcg/mL was achieved in pediatric patients 15 minutes after an intramuscular dose (10 mg/kg) of a 5% solution. Following rectal administration to pediatric patients, the onset of sleep occurs in 5 to 15 minutes. Plasma methohexital concentrations achieved following rectal administration tend to increase both with dose and with the use of more dilute solution concentrations when using the same dose. A 25 mg/kg dose of a 1% methohexital solution yielded plasma concentrations of 6.9 to 7.9 mcg/mL 15 minutes after dosing. The absolute bioavailability of rectal methohexital sodium is 17%.
With single doses, the rate of redistribution determines duration of pharmacologic effect. Metabolism occurs in the liver through demethylation and oxidation. Side-chain oxidation is the most important biotransformation involved in termination of biologic activity. Excretion occurs via the kidneys through glomerular filtration.
-
INDICATIONS AND USAGE
BREVITAL can be used in adults as follows:
1. For intravenous induction of anesthesia prior to the use of other general anesthetic agents.
2. For intravenous induction of anesthesia and as an adjunct to subpotent inhalational anesthetic agents (such as nitrous oxide in oxygen) for short surgical procedures; BREVITAL may be given by infusion or intermittent injection.
3. For use along with other parenteral agents, usually narcotic analgesics, to supplement subpotent inhalational anesthetic agents (such as nitrous oxide in oxygen) for longer surgical procedures.
4. As intravenous anesthesia for short surgical, diagnostic, or therapeutic procedures associated with minimal painful stimuli (see WARNINGS).
5. As an agent for inducing a hypnotic state.
BREVITAL can be used in pediatric patients older than 1 month as follows:
1. For rectal or intramuscular induction of anesthesia prior to the use of other general anesthetic agents.
2. For rectal or intramuscular induction of anesthesia and as an adjunct to subpotent inhalational anesthetic agents for short surgical procedures.
3. As rectal or intramuscular anesthesia for short surgical, diagnostic, or therapeutic procedures associated with minimal painful stimuli.
- CONTRAINDICATIONS
-
WARNINGS
As with all potent anesthetic agents and adjuncts, BREVITAL should be used only in hospital or ambulatory care settings that provide for continuous monitoring of respiratory (e.g. pulse oximetry) and cardiac function. Immediate availability of resuscitative drugs and age- and size-appropriate equipment for bag/valve/mask ventilation and intubation and personnel trained in their use and skilled in airway management should be assured. For deeply sedated patients, a designated individual other than the practitioner performing the procedure should be present to continuously monitor the patient.
Maintenance of a patent airway and adequacy of ventilation must be ensured during induction and maintenance of anesthesia with methohexital sodium solution. Laryngospasm is common during induction with all barbiturates and may be due to a combination of secretions and accentuated reflexes following induction or may result from painful stimuli during light anesthesia. Apnea/hypoventilation may be noted during induction, which may impair pulmonary ventilation; the duration of apnea may be longer than that produced by other barbiturate anesthetics. Cardiorespiratory arrest may occur.
This prescribing information describes intravenous use of methohexital sodium in adults. It also discusses intramuscular and rectal administration in pediatric patients older than one month. Although the published literature discusses intravenous administration in pediatric patients, the safety and effectiveness of intravenous administration of methohexital sodium in pediatric patients have not been established in well-controlled, prospective studies. (See PRECAUTIONS/Pediatric Use.)
Seizures may be elicited in subjects with a previous history of convulsive activity, especially partial seizure disorders.
Because the liver is involved in demethylation and oxidation of methohexital and because barbiturates may enhance preexisting circulatory depression, severe hepatic dysfunction, severe cardiovascular instability, or a shock-like condition may be reason for selecting another induction agent.
Prolonged administration may result in cumulative effects, including extended somnolence, protracted unconsciousness, and respiratory and cardiovascular depression. Respiratory depression in the presence of an impaired airway may lead to hypoxia, cardiac arrest, and death.
The CNS-depressant effect of BREVITAL may be additive with that of other CNS depressants, including ethyl alcohol and propylene glycol.
DANGER OF INTRA-ARTERIAL INJECTION—Unintended intra-arterial injection of barbiturate solutions may be followed by the production of platelet aggregates and thrombosis, starting in arterioles distal to the site of injection. The resulting necrosis may lead to gangrene, which may require amputation. The first sign in conscious patients may be a complaint of fiery burning that roughly follows the distribution path of the injected artery; if noted, the injection should be stopped immediately and the situation reevaluated. Transient blanching may or may not be noted very early; blotchy cyanosis and dark discoloration may then be the first sign in anesthetized patients. There is no established treatment other than prevention. The following should be considered prior to injection:
1. The extent of injury is related to concentration. Concentrations of 1% methohexital will usually suffice; higher concentrations should ordinarily be avoided.
2. Check the infusion to ensure that the catheter is in the lumen of a vein before injection.
Injection through a running intravenous infusion may enhance the possibility of detecting arterial placement; however, it should be remembered that the characteristic bright-red color of arterial blood is often altered by contact with drugs. The possibility of aberrant arteries should always be considered.
Post injury arterial injection of vasodilators and/or arterial infusion of parenteral fluids are generally regarded to be of no value in altering outcome. Animal experiments and published individual case reports concerned with a variety of arteriolar irritants, including barbiturates, suggest that 1 or more of the following may be of benefit in reducing the area of necrosis:
1. Arterial injection of heparin at the site of injury, followed by systemic anticoagulation.
2. Sympathetic blockade (or brachial plexus blockade in the arm).
3. Intra-arterial glucocorticoid injection at the site of injury, followed by systemic steroids.
4. A case report (nonbarbiturate injury) suggests that intra-arterial urokinase may promote fibrinolysis, even if administered late in treatment.
If extravasation is noted during injection of methohexital, the injection should be discontinued until the situation is remedied. Local irritation may result from extravasation; subcutaneous swelling may also serve as a sign of arterial or periarterial placement of the catheter.
Pediatric Neurotoxicity
Published animal studies demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity increase neuronal apoptosis in the developing brain and result in long-term cognitive deficits when used for longer than 3 hours. The clinical significance of these findings is not clear. However, based on the available data, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately three years of age in humans. (See PRECAUTIONS/Pregnancy, Pediatric Use, and ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY).
Some published studies in children suggest that similar deficits may occur after repeated or prolonged exposures to anesthetic agents early in life and may result in adverse cognitive or behavioral effects. These studies have substantial limitations, and it is not clear if the observed effects are due to the anesthetic/sedation drug administration or other factors such as the surgery or underlying illness.
Anesthetic and sedation drugs are a necessary part of the care of children needing surgery, other procedures, or tests that cannot be delayed, and no specific medications have been shown to be safer than any other. Decisions regarding the timing of any elective procedures requiring anesthesia should take into consideration the benefits of the procedure weighed against the potential risks.
-
PRECAUTIONS
General
All routes of administration of BREVITAL are often associated with hiccups, coughing, and/or muscle twitching, which may also impair pulmonary ventilation. Following induction, temporary hypotension and tachycardia may occur.
Recovery from methohexital anesthesia is rapid and smooth. The incidence of postoperative nausea and vomiting is low if the drug is administered to fasting patients. Postanesthetic shivering has occurred in a few instances.
The usual precautions taken with any barbiturate anesthetic should be observed with BREVITAL. The drug should be used with caution in patients with asthma, obstructive pulmonary disease, severe hypertension or hypotension, myocardial disease, congestive heart failure, severe anemia, or extreme obesity.
Methohexital sodium should be used with extreme caution in patients in status asthmaticus. Caution should be exercised in debilitated patients or in those with impaired function of respiratory, circulatory, renal, hepatic, or endocrine systems.
Information for Patients
Risk of Drowsiness
When appropriate, patients should be instructed as to the hazards of drowsiness that may follow use of BREVITAL. Outpatients should be released in the company of another individual, and no skilled activities, such as operating machinery or driving a motor vehicle, should be engaged in for 8 to 12 hours.
Effect of anesthetic and sedation drugs on early brain development
Studies conducted in young animals and children suggest repeated or prolonged use of general anesthetic or sedation drugs in children younger than 3 years may have negative effects on their developing brains. Discuss with parents and caregivers the benefits, risks, and timing and duration of surgery or procedures requiring anesthetic and sedation drugs (See WARNINGS/Pediatric Neurotoxicity).
Laboratory Tests
BSP and liver function studies may be influenced by administration of a single dose of barbiturates.
Drug Interactions
Prior chronic administration of barbiturates or phenytoin (e.g. for seizure disorder) appears to reduce the effectiveness of BREVITAL. Barbiturates may influence the metabolism of other concomitantly used drugs, such as phenytoin, halothane, anticoagulants, corticosteroids, ethyl alcohol, and propylene glycol-containing solutions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies to evaluate the carcinogenic potential of methohexital have not been conducted.
Pregnancy
Risk Summary
There are no adequate and well-controlled studies in pregnant women. In animal reproduction studies, no adverse developmental effects were observed following administration of methohexital to pregnant rabbits and rats during organogenesis at doses up to 4 and 7 times the human dose respectively.
Published studies in pregnant primates demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity during the period of peak brain development increases neuronal apoptosis in the developing brain of the offspring when used for longer than 3 hours. There are no data on pregnancy exposures in primates corresponding to periods prior to the third trimester in humans [See Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Reproduction studies have been performed in rabbits and rats at doses up to 4 and 7 times the human dose respectively and have revealed no evidence of harm to the fetus due to methohexital sodium.
In a published study in primates, administration of an anesthetic dose of ketamine for 24 hours on Gestation Day 122 increased neuronal apoptosis in the developing brain of the fetus. In other published studies, administration of either isoflurane or propofol for 5 hours on Gestation Day 120 resulted in increased neuronal and oligodendrocyte apoptosis in the developing brain of the offspring. With respect to brain development, this time period corresponds to the third trimester of gestation in the human. The clinical significance of these findings is not clear; however, studies in juvenile animals suggest neuroapoptosis correlates with long-term cognitive deficits. (See WARNINGS/Pediatric Neurotoxicity, PRECAUTIONS/Pediatric Use, and ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY).
Labor and Delivery
BREVITAL has been used in cesarean section delivery but, because of its solubility and lack of protein binding, it readily and rapidly traverses the placenta.
Pediatric Use
The safety and effectiveness of methohexital sodium in pediatric patients below the age of 1 month have not been established. Seizures may be elicited in subjects with a previous history of convulsive activity, especially partial seizure disorders. Apnea has been reported following dosing with methohexital regardless of the route of administration used. Studies using methohexital sodium intravenously in pediatric patients have been reported in the published literature. This literature is not adequate to establish the safety and effectiveness of intravenous administration of methohexital sodium in pediatric patients. Due to a variety of limitations such as study design, biopharmaceutic issues, and the wide range of effects observed with similar doses of intravenous methohexital, additional studies of intravenous methohexital in pediatric patients are necessary before this route can be recommended in pediatric patients. (See WARNINGS)
Published juvenile animal studies demonstrate that the administration of anesthetic and sedation drugs, such as BREVITAL, that either block NMDA receptors or potentiate the activity of GABA during the period of rapid brain growth or synaptogenesis, results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of ketamine that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer of isoflurane increased neuronal cell loss. Data from isoflurane-treated rodents and ketamine-treated primates suggest that the neuronal and oligodendrocyte cell losses are associated with prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in pregnant women, neonates, and young children who require procedures with the potential risks suggested by the nonclinical data. (See WARNINGS/ Pediatric Neurotoxicity, Pregnancy, and ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY).
Geriatric Use
Clinical studies of BREVITAL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Elderly subjects may commonly have conditions in which methohexital should be used cautiously such as obstructive pulmonary disease, severe hypertension or hypotension, preexisting circulatory depression, myocardial disease, congestive heart failure, or severe anemia. Caution should be exercised in debilitated patients or in those with impaired function of respiratory, circulatory, renal, hepatic, or endocrine systems (see WARNINGS, PRECAUTIONS and ADVERSE REACTIONS). Barbiturates may influence the metabolism of other concomitantly used drugs that are commonly taken by the elderly, such as anticoagulants and corticosteroids. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see PRECAUTIONS-Drug Interactions).
-
ADVERSE REACTIONS
Side effects associated with BREVITAL are extensions of pharmacologic effects and include:
Cardiovascular
Circulatory depression, thrombophlebitis, hypotension, tachycardia, peripheral vascular collapse, and convulsions in association with cardiorespiratory arrest
Respiratory
Respiratory depression (including apnea), cardiorespiratory arrest, laryngospasm, bronchospasm, hiccups, and dyspnea
Neurologic
Skeletal muscle hyperactivity (twitching), injury to nerves adjacent to injection site, and seizures
Psychiatric
Emergence delirium, restlessness, and anxiety may occur, especially in the presence of postoperative pain
Gastrointestinal
Nausea, emesis, abdominal pain, and liver function tests abnormal
Allergic
Erythema, pruritus, urticaria, and cases of anaphylaxis have been reported rarely
Other
Other adverse reactions include pain at injection site, salivation, headache, and rhinitis
For medical advice about adverse reactions contact your medical professional. To report SUSPECTED ADVERSE REACTIONS, contact Endo at 1-800-828-9393 or FDA at 1-800-FDA-1088 (1-800-332-1088) or www.fda.gov/medwatch/.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Signs and Symptoms
The onset of toxicity following an overdose of intravenously administered methohexital will be within seconds of the infusion. If methohexital is administered rectally or is ingested, the onset of toxicity may be delayed. The manifestations of an ultrashort-acting barbiturate in overdose include central nervous system depression, respiratory depression, hypotension, loss of peripheral vascular resistance, and muscular hyperactivity ranging from twitching to convulsive-like movements. Other findings may include convulsions and allergic reactions. Following massive exposure to any barbiturate, pulmonary edema, circulatory collapse with loss of peripheral vascular tone, and cardiac arrest may occur.
Treatment
To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians’ Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
Establish an airway and ensure oxygenation and ventilation. Resuscitative measures should be initiated promptly. For hypotension, intravenous fluids should be administered and the patient’s legs raised. If desirable increase in blood pressure is not obtained, vasopressor and/or inotropic drugs may be used as dictated by the clinical situation. For convulsions, diazepam intravenously and phenytoin may be required. If the seizures are refractory to diazepam and phenytoin, general anesthesia and paralysis with a neuromuscular blocking agent may be necessary.
Protect the patient’s airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient’s vital signs, blood gases, serum electrolytes, etc. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient’s airway when employing gastric emptying or charcoal.
-
DOSAGE AND ADMINISTRATION
Facilities for assisting ventilation and administering oxygen are necessary adjuncts for all routes of administration of anesthesia. Since cardiorespiratory arrest may occur, patients should be observed carefully during and after use of BREVITAL. Age- and size- appropriate resuscitative equipment (i.e., intubation and cardioversion equipment, oxygen, suction, and a secure intravenous line) and personnel qualified in its use must be immediately available.
Preanesthetic medication is generally advisable. BREVITAL may be used with any of the recognized preanesthetic medications.
Preparation of Solution
FOLLOW DILUTION INSTRUCTIONS EXACTLY.
Freshly prepare solutions of BREVITAL and use promptly. Reconstituted solutions of BREVITAL are chemically stable at room temperature for 24 hours.
Diluents
ONLY USE BACTERIOSTATIC-FREE DILUENT – Recommended diluents are based on route of administration (see Dilution Instructions).
Incompatible diluents: Lactated Ringer’s Injection
Dilution Instructions
1% solutions (10 mg/mL) should be prepared for intermittent intravenous and rectal administration; 0.2% solutions (2 mg/mL) should be prepared for continuous intravenous drug administration; 5% solutions (50 mg/mL) should be prepared for intramuscular administration.
Contents of vials should be diluted as follows:
FOR INTERMITTENT INTRAVENOUS and RECTAL ADMINISTRATION
The preferred diluent for intermittent intravenous and rectal administration is Sterile Water for Injection. 5% Dextrose Injection, or 0.9% Sodium Chloride Injection are also acceptable diluents.
Strength Amount of Diluent to Be Added to the Contents of the BREVITAL Vial For 1% methohexital solution (10 mg/mL) 500 mg
50 mL
no further dilution needed
FOR CONTINUOUS INTRAVENOUS ADMINISTRATION
For continuous drip anesthesia, prepare a 0.2% solution by adding 500 mg of BREVITAL Sodium to 250 mL of diluent. For this dilution, either 5% glucose solution or isotonic (0.9%) sodium chloride solution ONLY is recommended as the diluent instead of sterile water for injection in order to avoid extreme hypotonicity.
Strength Amount of Diluent to Be Added to the Contents of the BREVITAL Vial For 0.2% methohexital solution (2 mg/mL) 500 mg
15 mL
add to 235 mL diluent for 250 mL total volume
FOR INTRAMUSCULAR ADMINISTRATION
The preferred diluent for intramuscular administration is Sterile Water for Injection. 0.9% Sodium Chloride Injection is also an acceptable diluent.
Strength Amount of Diluent to Be Added to the Contents of the BREVITAL Vial For 5% methohexital solution (50 mg/mL) 500 mg
10 mL
no further dilution needed
Administration
Dosage is highly individualized; the drug should be administered only by those completely familiar with its quantitative differences from other barbiturate anesthetics.
Adults
BREVITAL is administered intravenously in a concentration of no higher than 1%. Higher concentrations markedly increase the incidence of muscular movements and irregularities in respiration and blood pressure.
Induction of anesthesia
For induction of anesthesia, a 1% solution is administered at a rate of about 1 mL/5 seconds. Gaseous anesthetics and/or skeletal muscle relaxants may be administered concomitantly. The dose required for induction may range from 50 to 120 mg or more but averages about 70 mg. The usual dosage in adults ranges from 1 to 1.5 mg/kg. The induction dose usually provides anesthesia for 5 to 7 minutes.
Maintenance of anesthesia
Maintenance of anesthesia may be accomplished by intermittent injections of the 1% solution or, more easily, by continuous intravenous drip of a 0.2% solution. Intermittent injections of about 20 to 40 mg (2 to 4 mL of a 1% solution) may be given as required, usually every 4 to 7 minutes. For continuous drip, the average rate of administration is about 3 mL of a 0.2% solution/minute (1 drop/second). The rate of flow must be individualized for each patient. For longer surgical procedures, gradual reduction in the rate of administration is recommended (see discussion of prolonged administration in WARNINGS). Other parenteral agents, usually narcotic analgesics, are ordinarily employed along with BREVITAL during longer procedures.
Pediatric Patients
BREVITAL is administered intramuscularly in a 5% concentration and administered rectally as a 1% solution.
Induction of anesthesia
For the induction of anesthesia by the intramuscular route of administration, the usual dose ranges from 6.6 to 10 mg/kg of the 5% concentration. For rectal administration, the usual dose for induction is 25 mg/kg using the 1% solution.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Compatibility Information
Solutions of BREVITAL should not be mixed in the same syringe or administered simultaneously during intravenous infusion through the same needle with acid solutions, such as atropine sulfate, metocurine iodide, and succinylcholine chloride. Alteration of pH may cause free barbituric acid to be precipitated. Solubility of the soluble sodium salts of barbiturates, including BREVITAL, is maintained only at a relatively high (basic) pH.
Because of numerous requests from anesthesiologists for information regarding the chemical compatibility of these mixtures, the following chart contains information obtained from compatibility studies in which a 1% solution of BREVITAL was mixed with therapeutic amounts of agents whose solutions have a low (acid) pH.
Active Ingredient Potency per mL Volume Used Immediate 15 min Physical Change
30 min1 h BREVITAL
10 mg
10 mL
CONTROL
Atropine Sulfate
1/150 gr
1 mL
None
Haze
Atropine Sulfate
1/100 gr
1 mL
None
Ppt
Ppt
Succinylcholine chloride
0.5 mg
4 mL
None
None
Haze
Succinylcholine chloride
1 mg
4 mL
None
None
Haze
Metocurine Iodide
0.5 mg
4 mL
None
None
Ppt
Metocurine Iodide
1 mg
4 mL
None
None
Ppt
Scopolamine Hydrobromide
1/120 gr
1 mL
None
None
None
Haze
Tubocurarine chloride
3 mg
4 mL
None
Haze
- HOW SUPPLIED
-
ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY
Published studies in animals demonstrate that the use of anesthetic agents during the period of rapid brain growth or synaptogenesis results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of an anesthetic regimen that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer increased neuronal cell loss. Data in rodents and in primates suggest that the neuronal and oligodendrocyte cell losses are associated with subtle but prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in neonates and young children who require procedures against the potential risks suggested by the nonclinical data. (See WARNINGS/Pediatric Neurotoxicity, PRECAUTIONS/Pregnancy, Pediatric Use).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1 × 500 mg Unit Carton
-
INGREDIENTS AND APPEARANCE
BREVITAL SODIUM
methohexital sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42023-105 Route of Administration INTRAMUSCULAR, INTRAVENOUS, RECTAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOHEXITAL SODIUM (UNII: 60200PNZ7Q) (METHOHEXITAL - UNII:E5B8ND5IPE) METHOHEXITAL SODIUM 500 mg Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CARBONATE (UNII: 45P3261C7T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42023-105-01 1 in 1 CARTON 11/01/2007 1 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA011559 11/01/2007 Labeler - Endo USA, Inc. (119185057)


