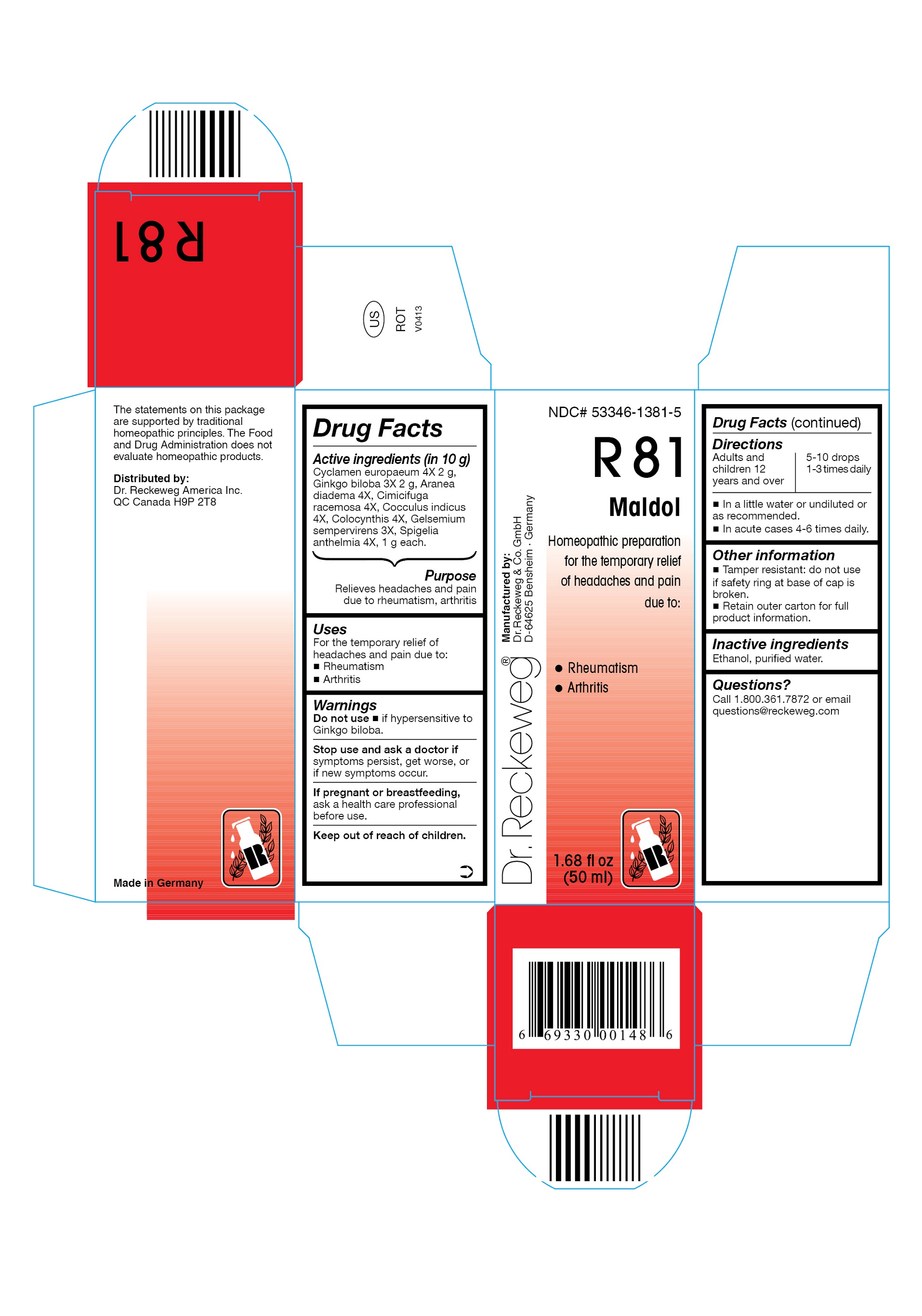
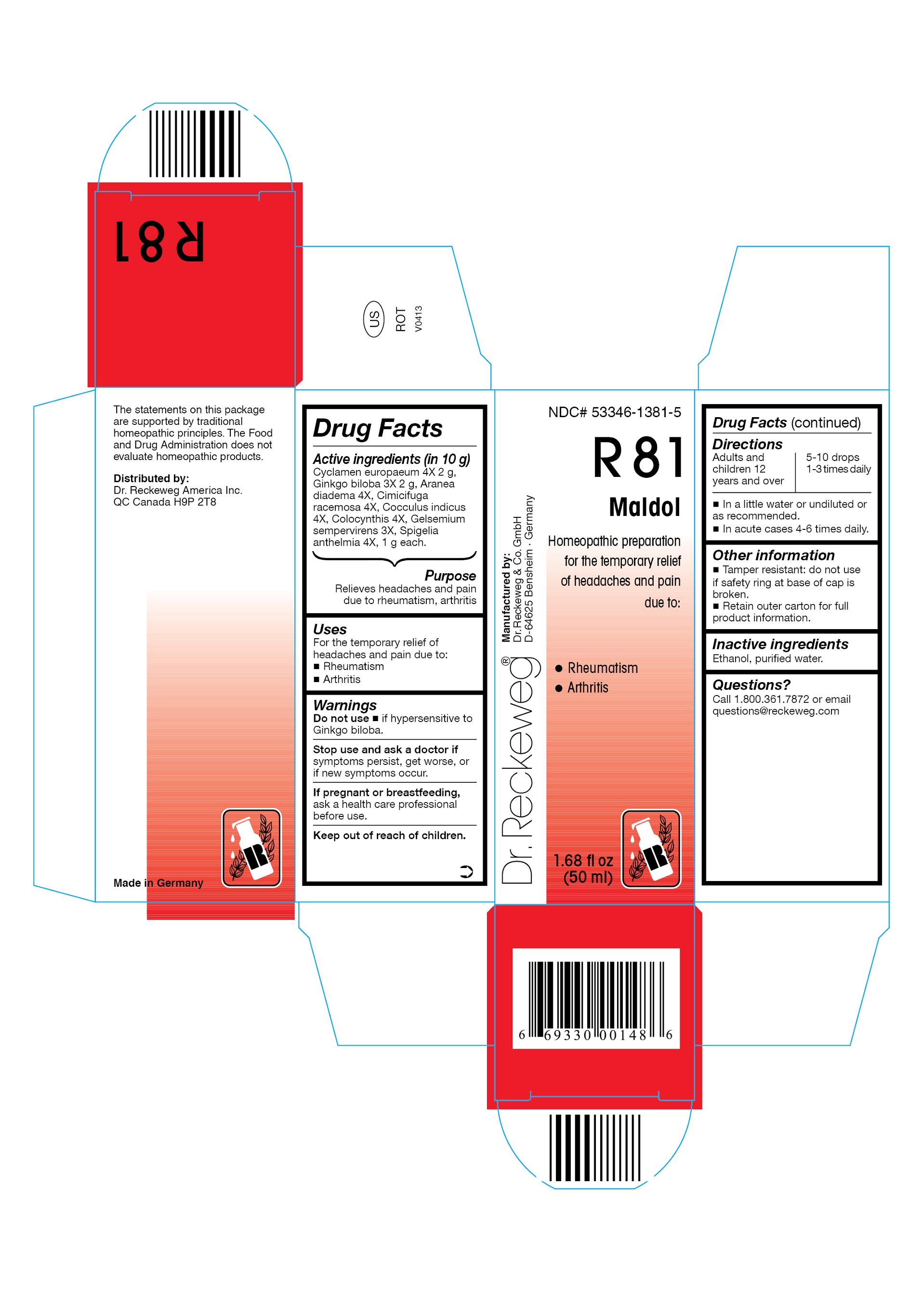
Label: DR. RECKEWEG R81 MALDOL COMBINATION PRODUCT- cyclamen europaeum 4x, ginkgo biloba 3x, aranea diadema 4x, cimicifuga racemosa 4x, cocculus indicus 4x, colocynthis 4x, gelsemium sempervirens 3x, spigelia anthelmia 4x liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 53346-1381-5 - Packager: PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 3, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR. RECKEWEG R81 MALDOL COMBINATION PRODUCT
cyclamen europaeum 4x, ginkgo biloba 3x, aranea diadema 4x, cimicifuga racemosa 4x, cocculus indicus 4x, colocynthis 4x, gelsemium sempervirens 3x, spigelia anthelmia 4x liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53346-1381 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLAMEN PURPURASCENS TUBER (UNII: G728143D8Q) (CYCLAMEN PURPURASCENS TUBER - UNII:G728143D8Q) CYCLAMEN PURPURASCENS TUBER 4 [hp_X] in 50 mL GINKGO (UNII: 19FUJ2C58T) (GINKGO - UNII:19FUJ2C58T) GINKGO 3 [hp_X] in 50 mL ARANEUS DIADEMATUS (UNII: 6T6CO7R3Z5) (ARANEUS DIADEMATUS - UNII:6T6CO7R3Z5) ARANEUS DIADEMATUS 4 [hp_X] in 50 mL BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 4 [hp_X] in 50 mL ANAMIRTA COCCULUS SEED (UNII: 810258W28U) (ANAMIRTA COCCULUS SEED - UNII:810258W28U) ANAMIRTA COCCULUS SEED 4 [hp_X] in 50 mL CITRULLUS COLOCYNTHIS FRUIT PULP (UNII: 23H32AOH17) (CITRULLUS COLOCYNTHIS FRUIT PULP - UNII:23H32AOH17) CITRULLUS COLOCYNTHIS FRUIT PULP 4 [hp_X] in 50 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 3 [hp_X] in 50 mL SPIGELIA ANTHELMIA (UNII: WYT05213GE) (SPIGELIA ANTHELMIA - UNII:WYT05213GE) SPIGELIA ANTHELMIA 4 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53346-1381-5 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/1986 Labeler - PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO (318602612) Establishment Name Address ID/FEI Business Operations PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO 318602612 manufacture(53346-1381)