Label: ZO SKIN HEALTH GEL SUNSCREEN BROAD-SPECTRUM SPF 50- avobenzone, octisalate, octocrylene gel
- NDC Code(s): 42851-092-45
- Packager: ZO Skin Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
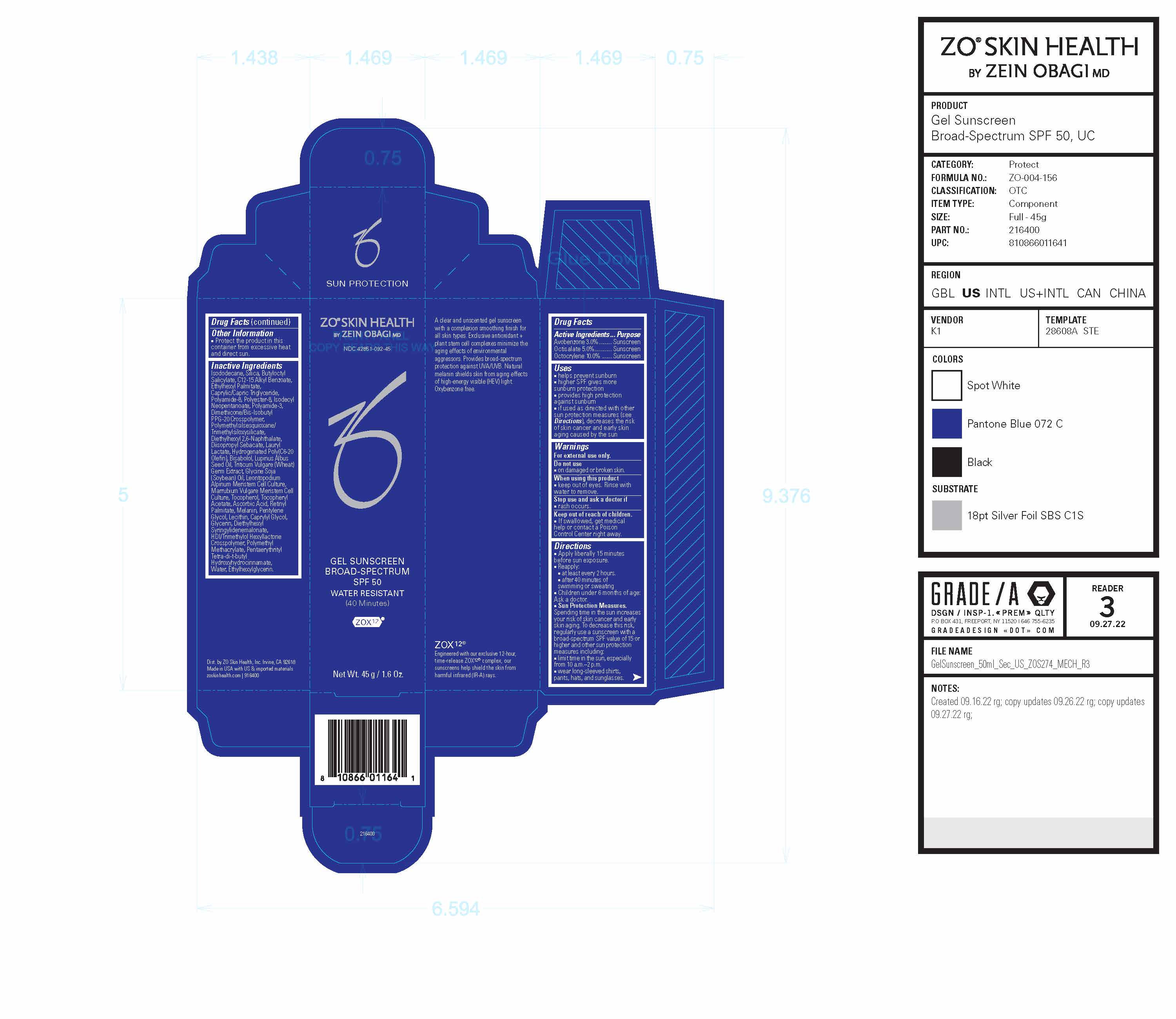
Drug Facts
Active Ingredients
Active Ingredients Purpose Avobenzone 3.0%......... Sunscreen Octisalate 5.0%............ Sunscreen Octocrylene 10.0% Sunscreen Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection provides high protection against sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply: ■ at least every 2 hours. ■ after 40 minutes of swimming or sweating
- Children under 6 months of age: Ask a doctor.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF value of 15 or higher and other sun protection measures including: ■ limit time in the sun, especially from 10 a.m.–2 p.m. ■ wear long-sleeved shirts, pants, hats, and sunglasses.
Inactive Ingredients
Isododecane, Silica, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Ethylhexyl Palmitate, Caprylic/Capric Triglyceride, Polyamide-8, Polyester-8, Isodecyl Neopentanoate, Polyamide-3, Dimethicone/Bis-Isobutyl PPG-20 Crosspolymer, Polymethylsilsesquioxane/Trimethylsiloxysilicate, Diethylhexyl 2,6-Naphthalate, Diisopropyl Sebacate, Lauryl Lactate, Hydrogenated Poly(C6-20 Olefin), Bisabolol, Lupinus Albus Seed Oil, Triticum Vulgare (Wheat) Germ Extract, Glycine Soja (Soybean) Oil, Leontopodium Alpinum Meristem Cell Culture, Marrubium Vulgare Meristem Cell Culture, Tocopherol, Tocopheryl Acetate, Ascorbic Acid, Retinyl Palmitate, Melanin, Pentylene Glycol, Lecithin, Caprylyl Glycol, Glycerin, Diethylhexyl Syringylidenemalonate, HDI/Trimethylol Hexyllactone Crosspolymer, Polymethyl Methacrylate, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Water, Ethylhexylglycerin.
-
Principal Display Panel
ZO® Skin Health Gel Sunscreen Broad-Spectrum SPF 50
Water-Resistant
(40 Minutes)
ZOX12®
Engineered with our exclusive 12-hour, time-release ZOX12® complex, our sunscreens help shield the skin from harmful infrared (IR-A) rays.
A clear and unscented gel sunscreen with a complexion smoothing finish for all skin types. Exclusive antioxidant + plant stem cell complexes minimize the aging effects of environmental aggressors. Provides broad-spectrum protection against UVA/UVB. Natural melanin shields skin from aging effects of high-energy visible (HEV) light. Oxybenzone free.
Dist. by ZO Skin Health, Inc. Irvine, CA 92618
Made in USA with US & imported materials
zoskinhealth.com
-
INGREDIENTS AND APPEARANCE
ZO SKIN HEALTH GEL SUNSCREEN BROAD-SPECTRUM SPF 50
avobenzone, octisalate, octocrylene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42851-092 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4.5 g in 45 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.35 g in 45 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 2.25 g in 45 g Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISODODECANE (UNII: A8289P68Y2) POLYAMIDE-8 (4500 MW) (UNII: 77723GV81A) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) WATER (UNII: 059QF0KO0R) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) TOCOPHEROL (UNII: R0ZB2556P8) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) WHEAT GERM (UNII: YR3G369F5A) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) GLYCERIN (UNII: PDC6A3C0OX) DIETHYLHEXYL 2,6-NAPHTHALATE (UNII: I0DQJ7YGXM) POLYAMIDE-3 (12000 MW) (UNII: L7P3YWF22X) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) POLYMETHYLSILSESQUIOXANE/TRIMETHYLSILOXYSILICATE (UNII: X2PZH4Y6HT) LAURYL LACTATE (UNII: G5SU0BFK7O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PENTYLENE GLYCOL (UNII: 50C1307PZG) LUPINUS ALBUS SEED OIL (UNII: 958BJW095Q) SOYBEAN OIL (UNII: 241ATL177A) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYDROGENATED C6-20 POLYOLEFIN (100 CST) (UNII: 39EYQ1W9RB) LEVOMENOL (UNII: 24WE03BX2T) ASCORBIC ACID (UNII: PQ6CK8PD0R) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-092-45 1 in 1 CARTON 04/01/2023 1 45 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 04/01/2023 Labeler - ZO Skin Health, Inc. (826468527)

