Label: AQUASOL A- vitamin a palmitate injection, solution
- NDC Code(s): 70199-026-11
- Packager: Casper Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 14, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
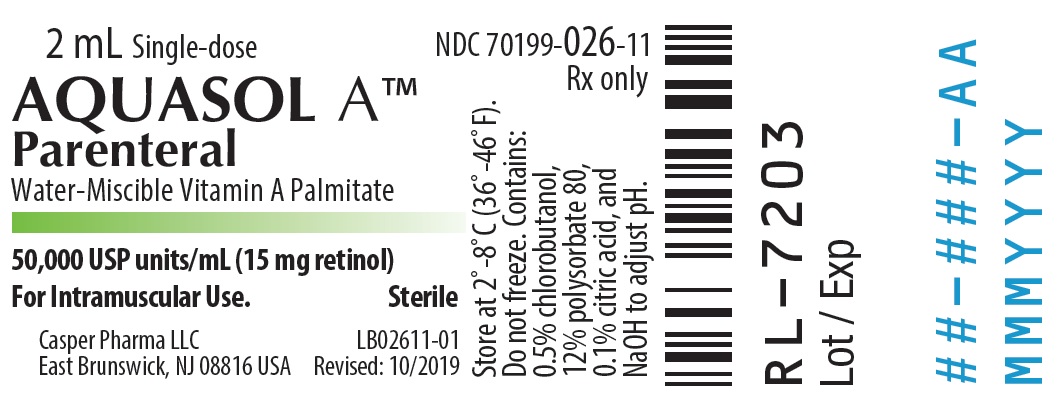
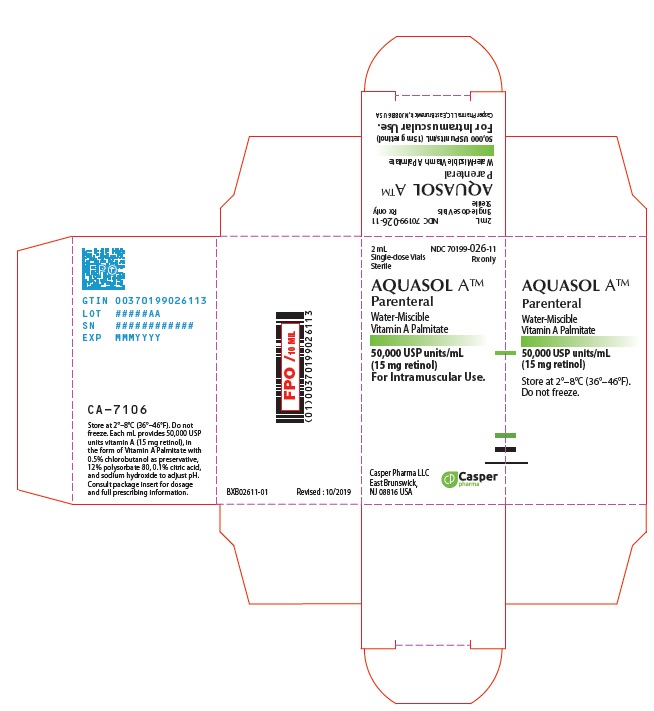
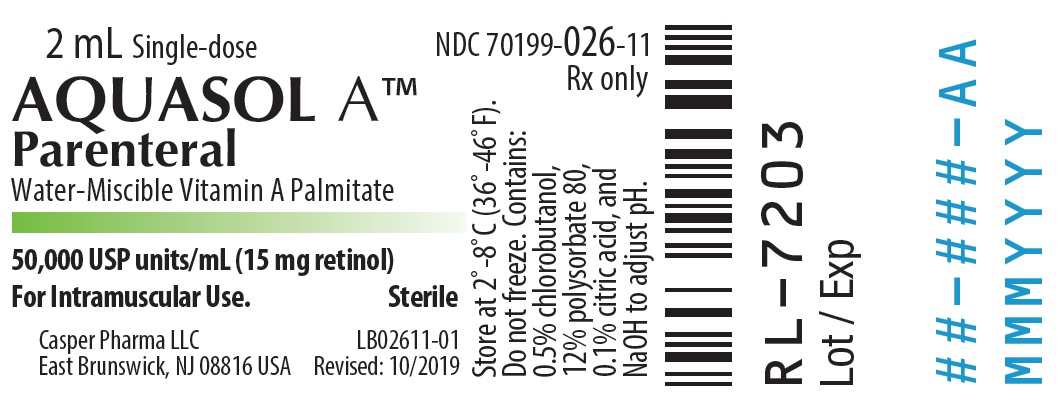
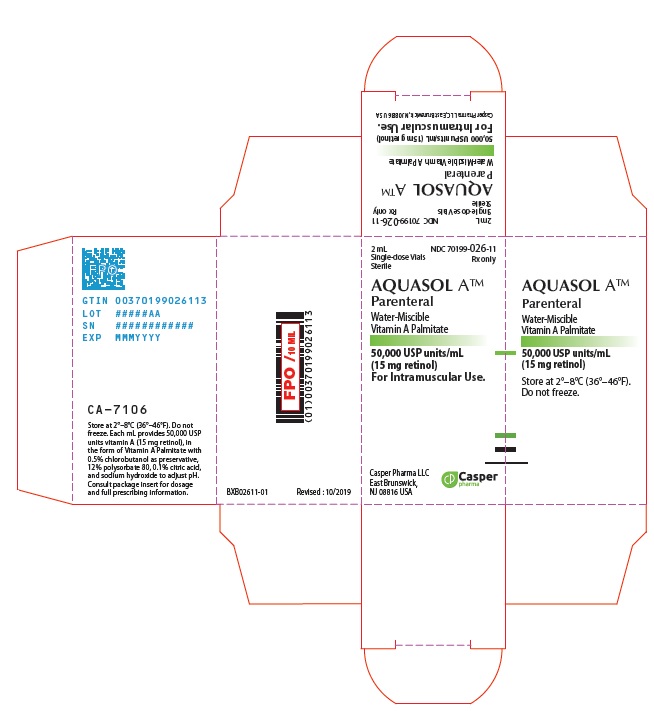
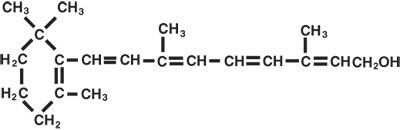
AQUASOL ATM Parenteral (water-miscible vitamin A Palmitate) provides 50,000 USP Units of vitamin A per mL as retinol (C20H30O) in the form of vitamin A palmitate, a light yellow to amber oil. Contains 0.5% chlorobutanol as preservative; 12% polysorbate 80, 0.1% citric acid, and sodium hydroxide to adjust pH. The structural formula of retinol is:
Ordinarily oil-soluble, the vitamin A in this product has been water solubilized by special processing* and is available in a water solution for intramuscular injection.
One USP Unit is equivalent to one international unit (IU) and to 0.3 mcg of retinol or 0.6 mcg of betacarotene.
-
CLINICAL PHARMACOLOGY
Beta-carotene, retinol, and retinal have effective and reliable vitamin A activity. Retinal and retinol are in chemical equilibrium in the body and have equivalent antixerophthalmic activity. Retinal combines with the rod pigment, opsin, in the retina to form rhodopsin, necessary for visual dark adaptation. Vitamin A prevents retardation of growth and preserves the epithelial cells' integrity. Normal adult liver storage is sufficient to satisfy two years' requirements of vitamin A.
Vitamin A is readily absorbed from the gastrointestinal tract, where the biosynthesis of vitamin A from beta-carotene takes place. Vitamin A absorption requires bile salts, pancreatic lipase, and dietary fat. It is transported in the blood to the liver by the chylomicron fraction of the lymph. Vitamin A is stored in Kupffer cells of the liver mainly as the palmitate. Normal serum vitamin A is 80–300 Units per 100 mL (plasma range is 30–70 mcg per dl) and for carotenoids 270–753 Units per 100 mL. The normal adult liver contains approximately 100 to 300 micrograms per gram, mostly as retinol palmitate.
*Oil-soluble vitamin A water solubilized with polysorbate 80.
-
INDICATIONS
Vitamin A injection is effective for the treatment of vitamin A deficiency.
The parenteral administration is indicated when the oral administration is not feasible as in anorexia, nausea, vomiting, pre- and postoperative conditions, or it is not available as in the "Malabsorption Syndrome" with accompanying steatorrhea.
Pediatric Use: Vitamin A treatment for deficiency states has been recognized as an especially effective and important therapy in the pediatric population.
Vitamin A supplementation for deficiency states in this population has been addressed by the Committee on Clinical Practice Issues of the American Society for Clinical Nutrition, by the American Society for Parenteral and Enteral Nutrition, and by the World Health Organization.
-
CONTRAINDICATIONS
The intravenous administration. Hypervitaminosis A. Sensitivity to any of the ingredients in this preparation.
Use in Pregnancy: Safety of amounts exceeding 6,000 Units of vitamin A daily during pregnancy has not been established at this time. The use of vitamin A in excess of the recommended dietary allowance may cause fetal harm when administered to a pregnant woman. Animal reproduction studies have shown fetal abnormalities associated with overdosage in several species. Malformations of the central nervous system, the eye, the palate, and the urogenital tract are recorded. Vitamin A in excess of the recommended dietary allowance is contraindicated in women who are or may become pregnant. If vitamin A is used during pregnancy, or if the patient becomes pregnant while taking vitamin A, the patient should be apprised of the potential hazard to the fetus.
- WARNINGS
-
PRECAUTIONS
General: Protect from light. Prolonged daily dose administration over 25,000 Units vitamin A should be under close supervision. Blood level assays are not a direct measure of liver storage. Liver storage should be adequate before discontinuing therapy. Single vitamin A deficiency is rare. Multiple vitamin deficiency is expected in any dietary deficiency.
Drug Interactions: Women on oral contraceptives have shown a significant increase in plasma vitamin A levels.
Carcinogenesis: There are no studies that show that administration of vitamin A will cause or prevent cancer.
Pregnancy:
See CONTRAINDICATIONS section.
Nursing Mothers: The U.S. Recommended Daily Allowance (RDA) of vitamin A (5,000 Units) is recommended for nursing mothers.
-
ADVERSE REACTIONS
See OVERDOSAGE section. Anaphylactic shock and death have been reported using the intravenous route. Allergic reactions have been reported rarely with administration of AQUASOL ATM Parenteral including one case of an anaphylactoid type reaction.
-
OVERDOSAGE
The following amounts have been found to be toxic orally. Toxicity manifestations depend on the age, dosage, size, and duration of administration.
Acute toxicity — single dose (25,000 Units/kg body weight)
Infant: 350,000 Units
Adult: Over 2 million UnitsChronic toxicity (4,000 Units/kg body weight for 6 to 15 months)
Infants 3 to 6 months old: 18,500 Units (water dispersed)/day for 1 to 3 months.
Adult: 1 million Units daily for three days; 50,000 Units daily for longer than 18 months; 500,000 Units daily for two months.Hypervitaminosis A Syndrome:
- 1.
-
General manifestations:
Fatigue, malaise, lethargy, abdominal discomfort, anorexia, and vomiting. - 2.
- Specific manifestations:
- a. Skeletal: hepatotoxicity, hard tender cortical thickening over the radius and tibia, migratory arthralgia, slow growth, and premature closure of the epiphysis leading to arrested bone growth in children.
b. Central Nervous System: irritability, headache, and increased intracranial pressure as manifested by bulging fontanels, papilledema, and exophthalmos.
c. Dermatologic: fissures of the lips, drying and cracking of the skin, alopecia, scaling, massive desquamation, and increased pigmentation.
d. Systemic: hypomenorrhea, hepatosplenomegaly, hepatotoxicity, jaundice, leukopenia, vitamin A plasma level over 1,200 Units/100 mL.
The treatment of hypervitaminosis A consists of immediate withdrawal of the vitamin along with symptomatic and supportive treatment.
-
DOSAGE AND ADMINISTRATION
For intramuscular use.
- I.
- Adults
100,000 Units daily for three days followed by 50,000 Units daily for two weeks. - II.
- Pediatric patients 1 to 8 years old
17,500 to 35,000 Units daily for 10 days. - III.
- Infants
7,500 to 15,000 Units daily for 10 days.
Follow-up therapy with an oral therapeutic multivitamin preparation, containing 10,000 to 20,000 Units vitamin A for adults and for pediatric patients over 8 years old, and 5,000 to 10,000 Units for infants and other pediatric patients under 8 years old, is recommended daily for two months. Low birth-weight infants may require additional vitamin A though the exact dosing in these pediatric patients has not been established. In malabsorption, the parenteral route must be used for an equivalent preparation.
Poor dietary habits should be corrected and an abundant and well-balanced dietary intake should be prescribed.
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 2 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 2 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
AQUASOL A
vitamin a palmitate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70199-026 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) (RETINOL - UNII:G2SH0XKK91) RETINOL 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength CHLOROBUTANOL (UNII: HM4YQM8WRC) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70199-026-11 1 in 1 CARTON 10/01/2020 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA006823 10/01/2020 Labeler - Casper Pharma LLC (080025838)