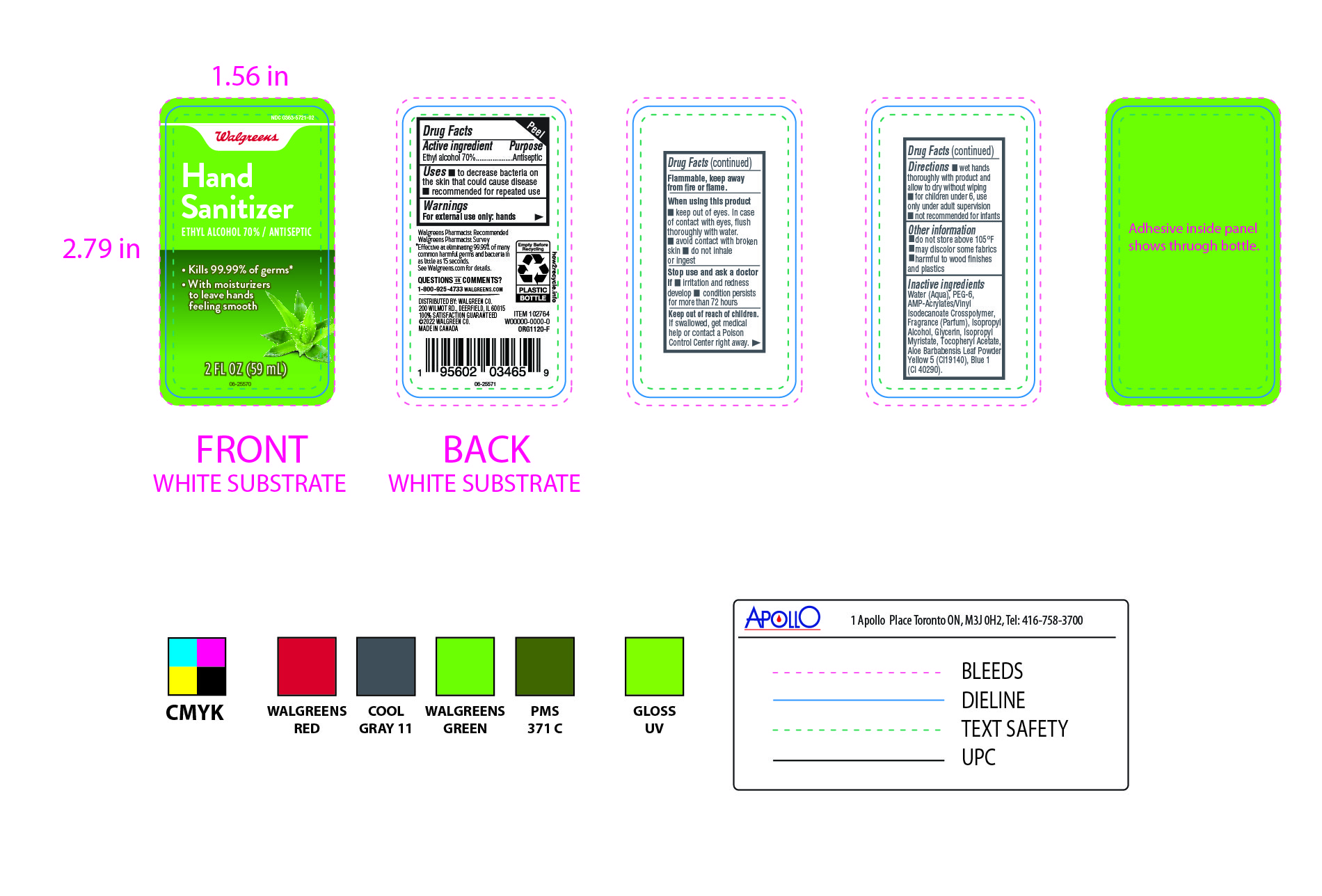
Label: WALGREENS- ethyl alcohol gel
- NDC Code(s): 0363-5721-02
- Packager: walgreens
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WALGREENS
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-5721 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 700 mg in 1 mL Inactive Ingredients Ingredient Name Strength FRAGRANCE CLEAN ORC0600327 (UNII: 329LCV5BTF) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) BASIC YELLOW 5 (UNII: 07BP340B4T) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) CORN OIL PEG-6 ESTERS (UNII: 1U4409FY0P) WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) DIRECT BLUE 1 (UNII: 8NN34MAQ6H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-5721-02 59 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/02/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 11/02/2022 Labeler - walgreens (008965063) Registrant - Apollo Health and Beauty Care (201901209) Establishment Name Address ID/FEI Business Operations Apollo Health and Beauty Care 201901209 manufacture(0363-5721)