Label: FAMOTIDINE- famotidine tablet, film coated
-
NDC Code(s):
69230-326-01,
69230-326-05,
69230-326-10,
69230-326-30, view more69230-326-60, 69230-327-01, 69230-327-05, 69230-327-10, 69230-327-30, 69230-327-50
- Packager: Camber Consumer Care Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 11, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
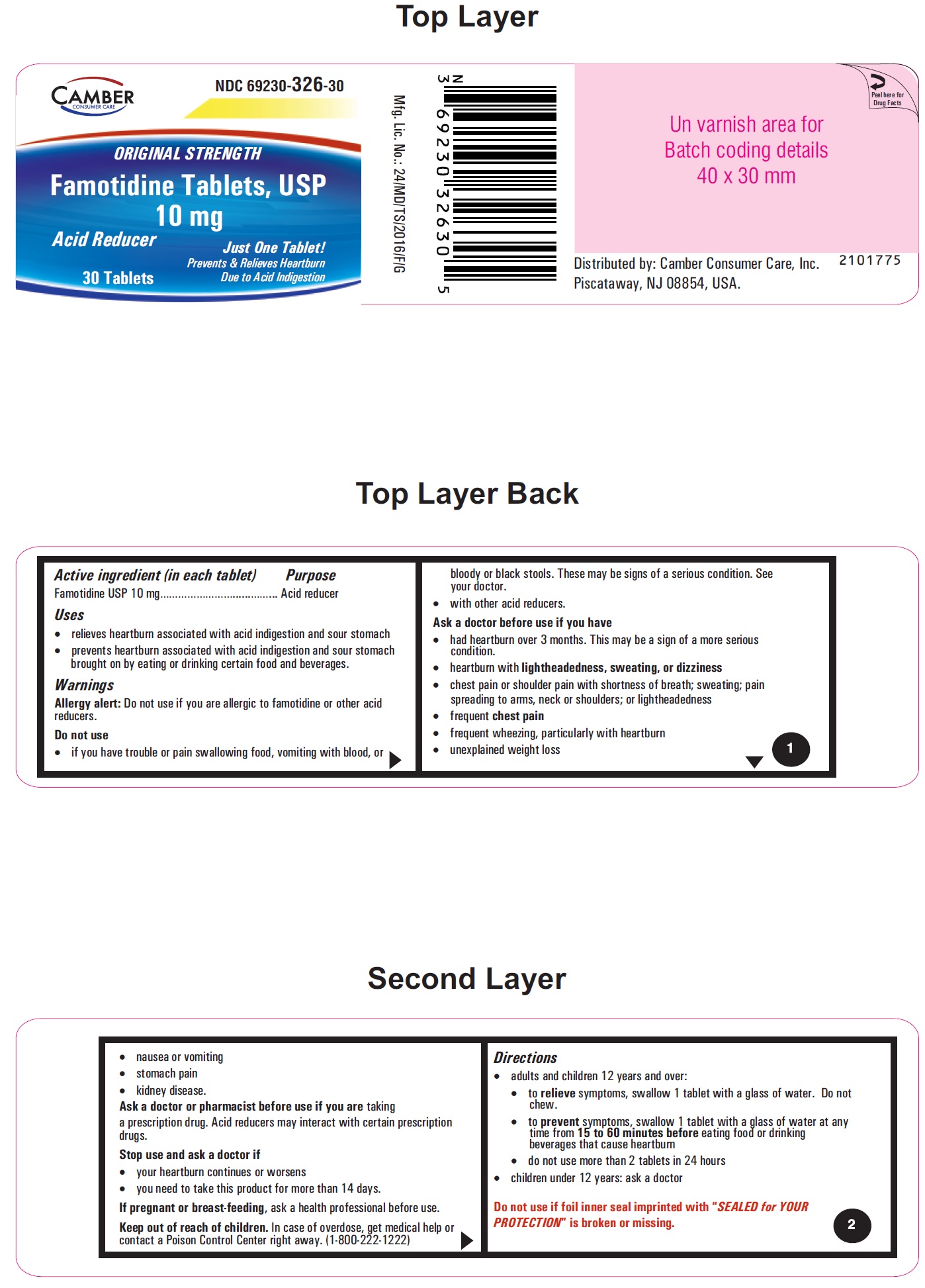
- Active ingredient (in each tablet)
- Purpose
- Uses
- Warnings
- Do not use
-
Ask a doctor before use if you have
• had heartburn over 3 months. This may be a sign of a more serious condition.
• heartburn with lightheadedness, sweating, or dizziness
• chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
• frequent chest pain
• frequent wheezing, particularly with heartburn
• unexplained weight loss
• nausea or vomiting
• stomach pain
• kidney disease - Ask a doctor or pharmacist before use if you are
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
• adults and children 12 years and over:
• to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.10 mg:
• to prevent symptoms, swallow 1 tablet with a glass of water at any time from 15 to 60 minutes before eating food or drinking beverages that cause heartburn20 mg:
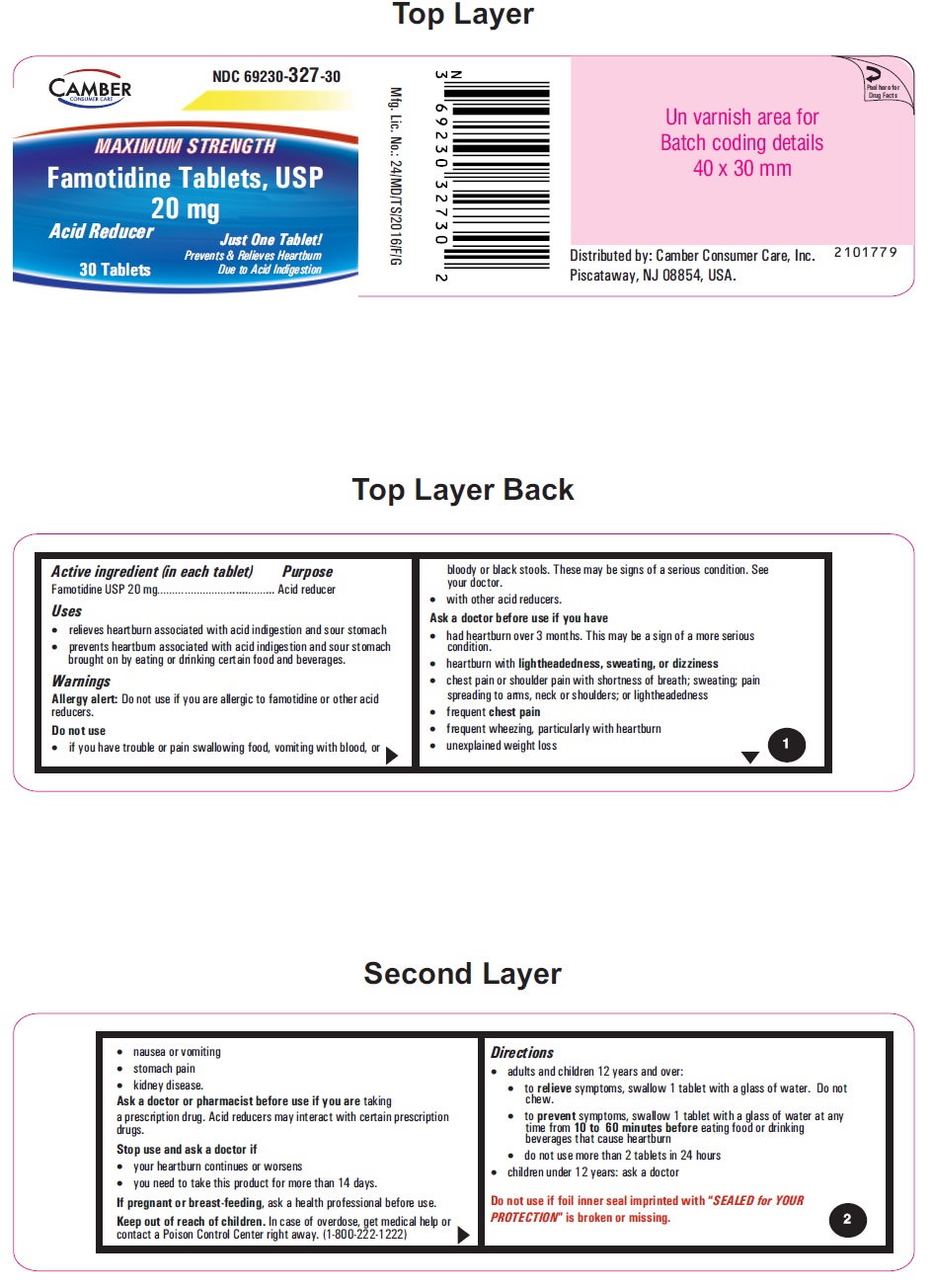
• to prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn
• do not use more than 2 tablets in 24 hours
• children under 12 years: ask a doctor - Other information
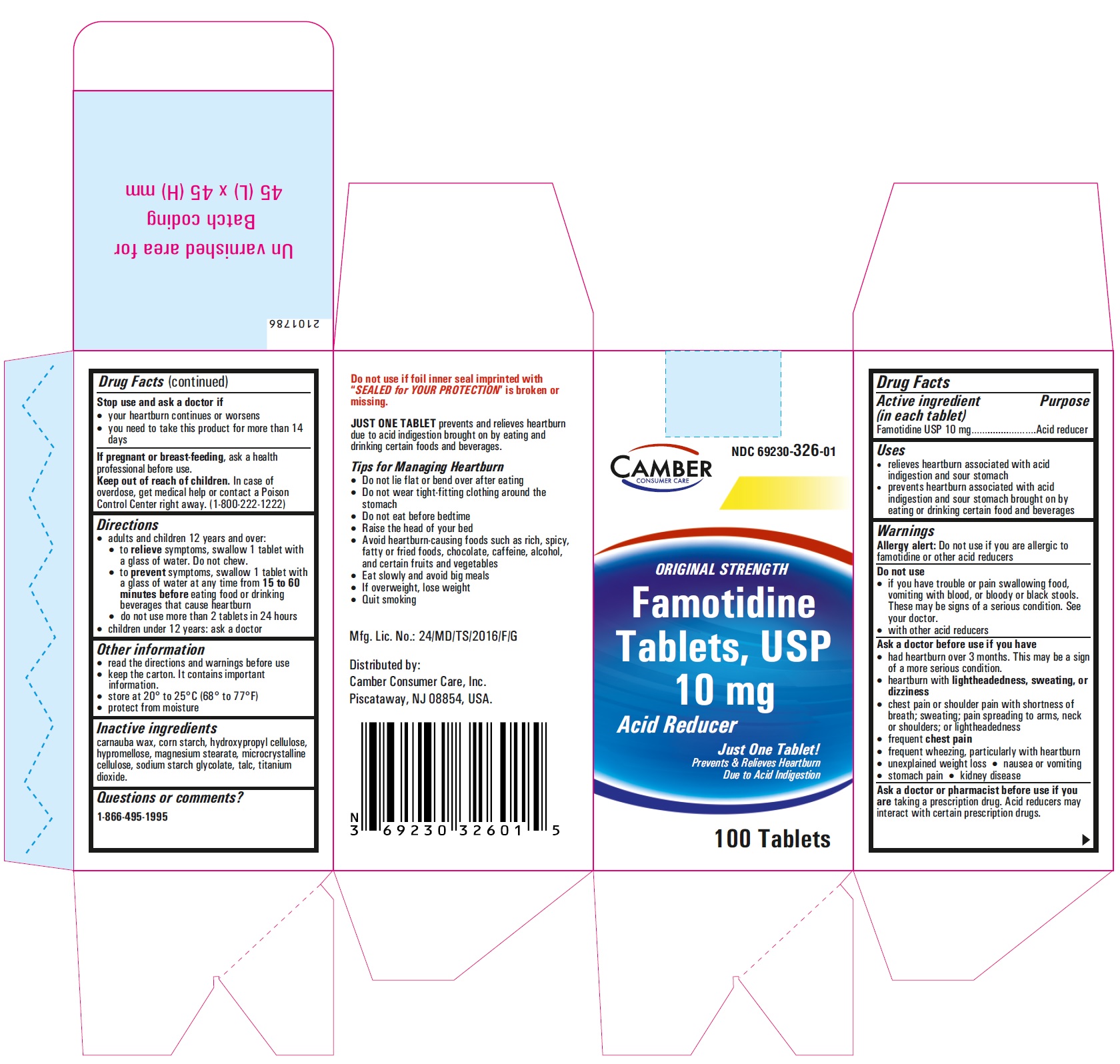
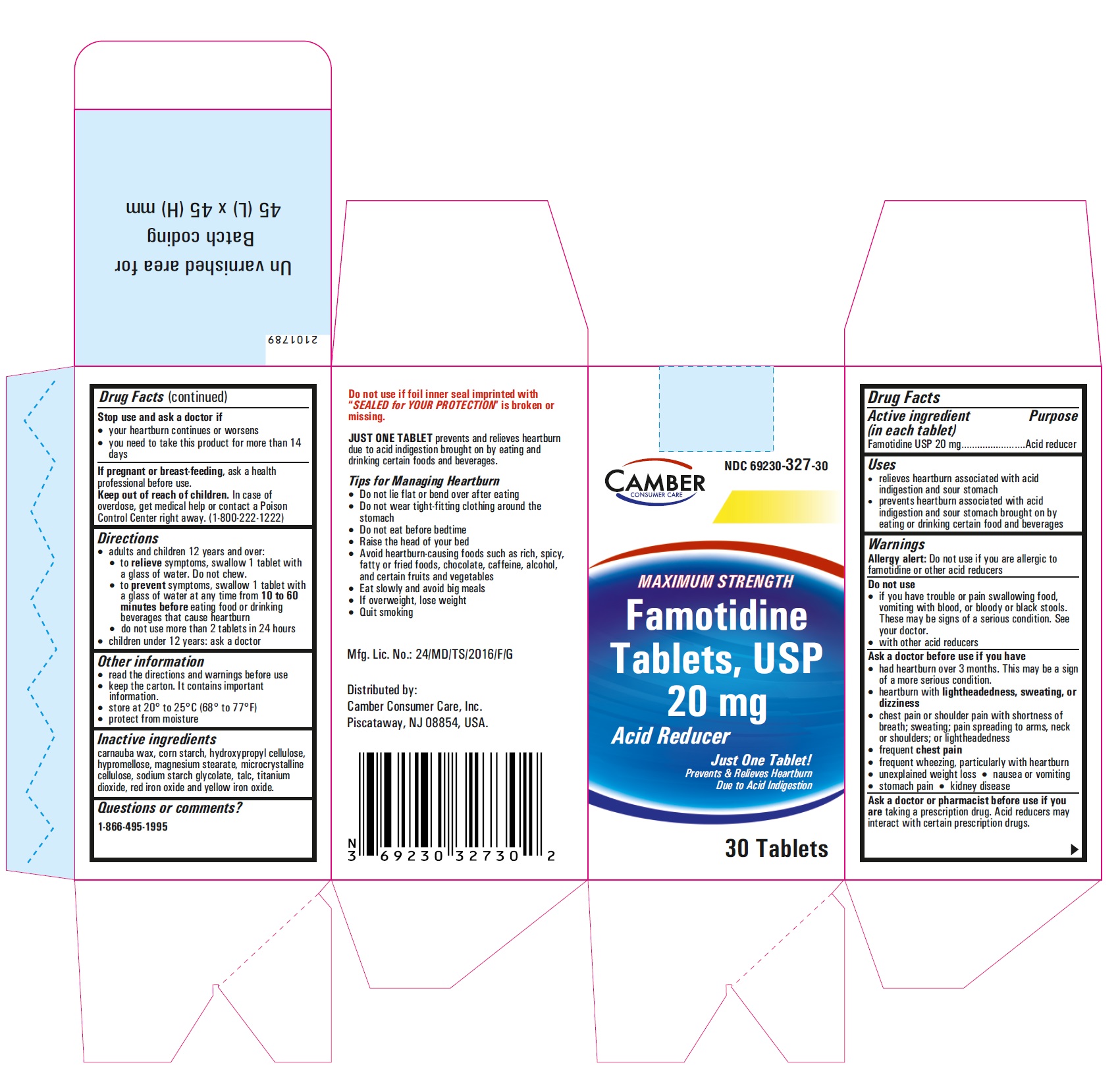
- Inactive ingredients
- Questions or comments?
-
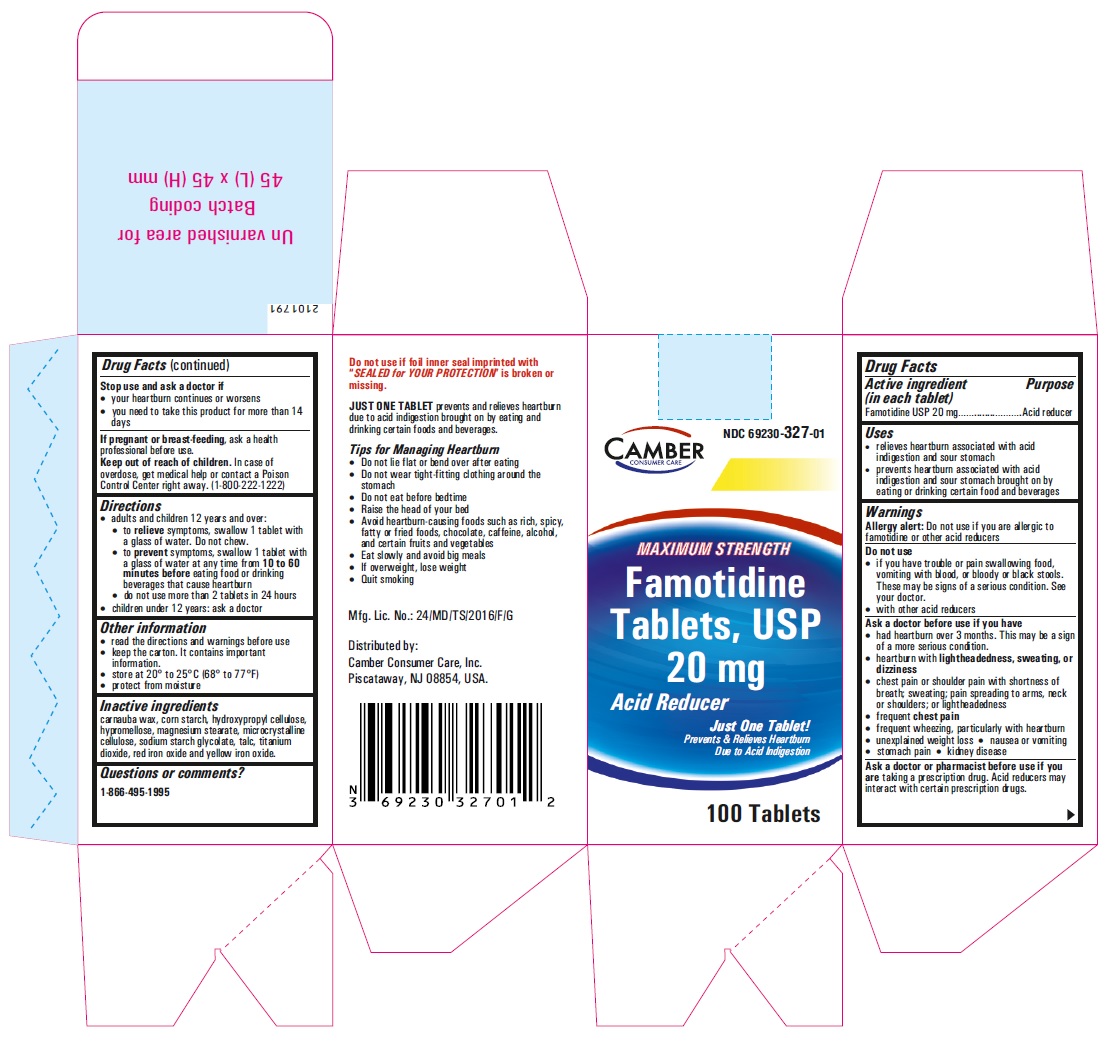
PRINCIPAL DISPLAY PANEL
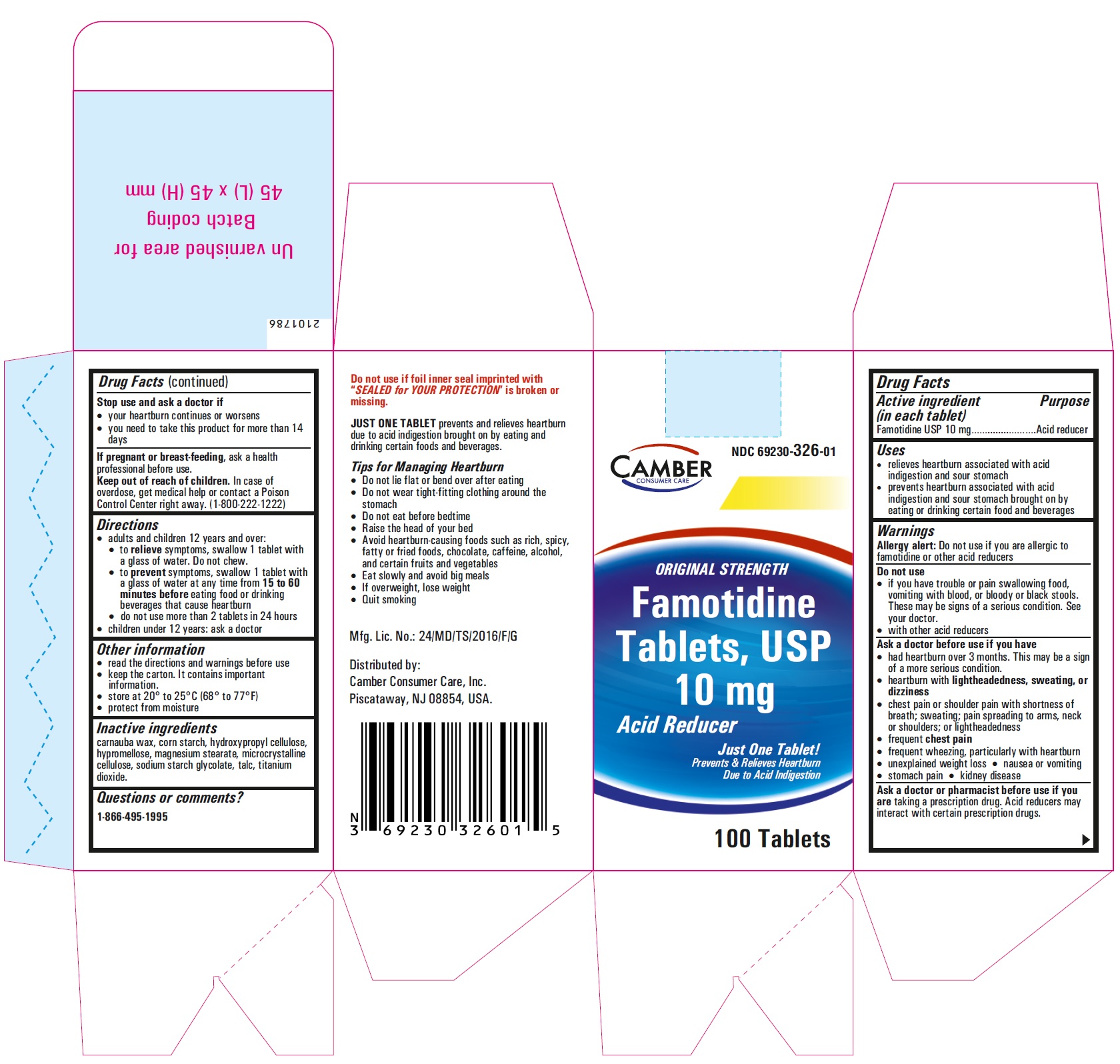
Famotidine Tablets USP 10 mg - 30s container label
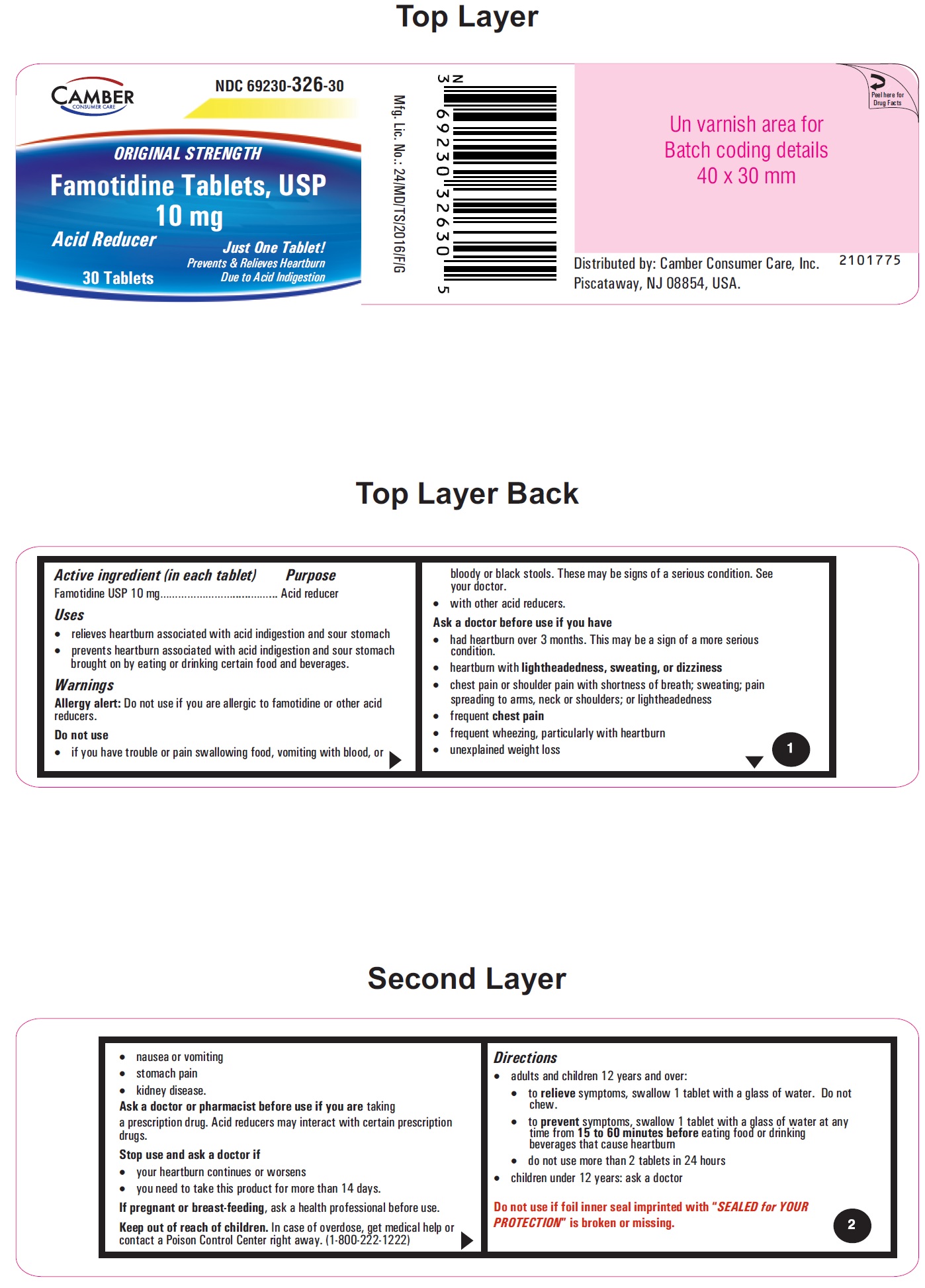
Famotidine Tablets USP 10 mg - 30s container carton label

Famotidine Tablets USP 10 mg - 10 (1x10) blister carton label
Famotidine Tablets USP 20 mg - 30s container label
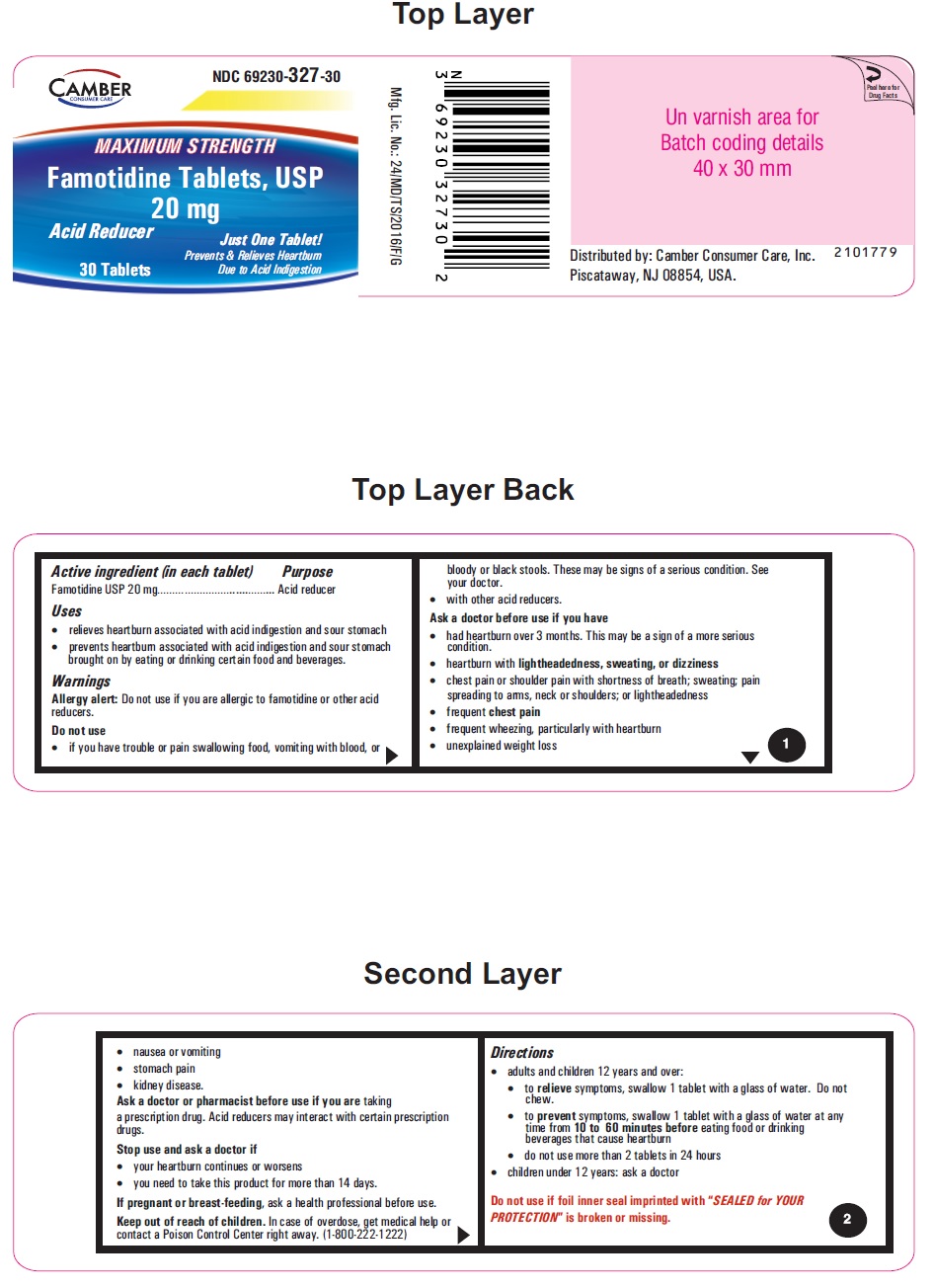
Famotidine Tablets USP 20 mg - 30s container carton label
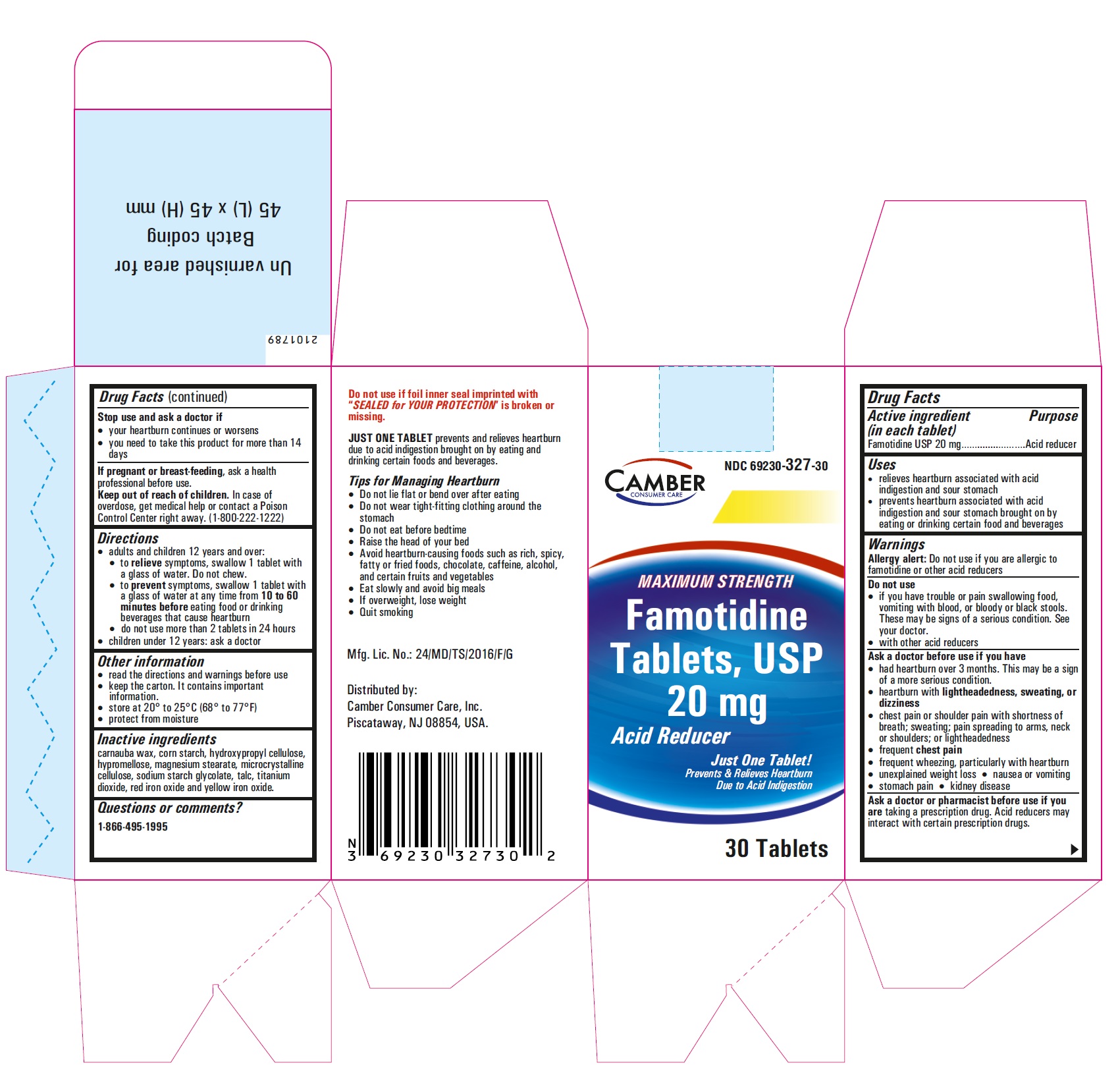
Famotidine Tablets USP 20 mg - 10 (1x10) blister carton label
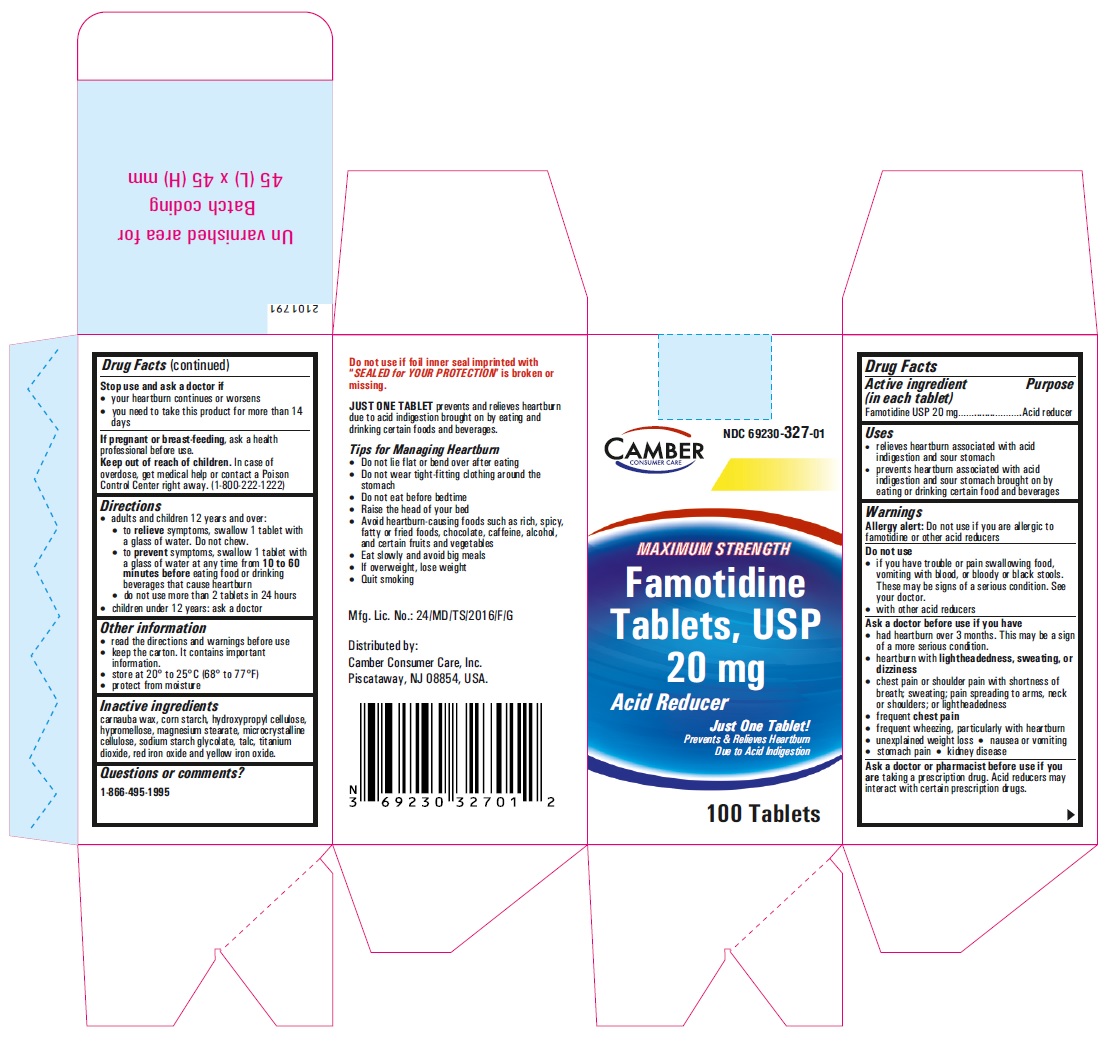
-
INGREDIENTS AND APPEARANCE
FAMOTIDINE
famotidine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69230-326 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 10 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code T;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69230-326-30 1 in 1 CARTON 11/08/2021 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:69230-326-60 1 in 1 CARTON 11/08/2021 2 60 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:69230-326-01 1 in 1 CARTON 11/08/2021 3 100 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:69230-326-05 1 in 1 CARTON 11/08/2021 4 500 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:69230-326-10 1 in 1 CARTON 11/08/2021 5 1000 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215766 11/08/2021 FAMOTIDINE
famotidine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69230-327 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 20 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) CARNAUBA WAX (UNII: R12CBM0EIZ) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color yellow (LIGHT YELLOW) Score no score Shape ROUND Size 6mm Flavor Imprint Code T;11 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69230-327-30 1 in 1 CARTON 11/08/2021 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:69230-327-50 1 in 1 CARTON 11/08/2021 2 50 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:69230-327-01 1 in 1 CARTON 11/08/2021 3 100 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:69230-327-05 1 in 1 CARTON 11/08/2021 4 500 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:69230-327-10 1 in 1 CARTON 11/08/2021 5 1000 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215766 11/08/2021 Labeler - Camber Consumer Care Inc (079539968) Establishment Name Address ID/FEI Business Operations Annora Pharma Private Limited 650980746 analysis(69230-326, 69230-327) , manufacture(69230-326, 69230-327)