Label: FLUORESCITE- fluorescein sodium injection, solution
- NDC Code(s): 0065-0092-05, 0065-0092-65
- Packager: Alcon, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FLUORESCITE® safely and effectively.
See full prescribing information for FLUORESCITE® (fluorescein injection, USP) 10%.
FLUORESCITE® (fluorescein injection, USP) 10% Intravenous injection
Initial U.S. Approval: 1976
INDICATIONS AND USAGE
FLUORESCITE® Injection 10% is indicated in diagnostic fluorescein angiography or angioscopy of the retina and iris vasculature. (1)
DOSAGE AND ADMINISTRATION
The normal adult dose of FLUORESCITE® Injection 10% (100 mg/mL) is 500 mg via intravenous administration. (2.1)
For children, the dose should be calculated on the basis of 7.7 mg for each kg of actual body weight (or 35 mg for each 10 pounds of body weight) up to a maximum of 500 mg via intravenous administration. (2.1)
DOSAGE FORMS AND STRENGTHS
Single use 5 mL vial containing 100 mg/mL fluorescein. (3)
CONTRAINDICATIONS
FLUORESCITE® Injection 10% is contraindicated in patients with known hypersensitivity to fluorescein sodium or any other ingredients in this product (4.1)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions include skin discoloration, urine discoloration, nausea, vomiting, and gastrointestinal distress. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Alcon Laboratories,Inc. at 1-800-757-9195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Caution should be exercised when fluorescein sodium is administered to a nursing woman. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing
2.2 Preparation for Administration
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Respiratory Reactions
5.2 Severe Local Tissue Damage
5.3 Nausea and/or Vomiting
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing
Adult Dose- The normal adult dose of FLUORESCITE® Injection 10% (100 mg/mL) is 500 mg via intravenous administration.
For children, the dose should be calculated on the basis of 7.7 mg for each kg of actual body weight (or 35 mg for each 10 pounds of body weight) up to a maximum of 500 mg via intravenous administration.
2.2 Preparation for Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not mix or dilute with other solutions or drugs. Flush intravenous cannulas before and after drugs are injected to avoid physical incompatibility reactions.
2.3 Administration
Inject the dose rapidly (1 mL per second is normally recommended) intravenously into the antecubital vein, after taking precautions to avoid extravasation. A syringe, filled with FLUORESCITE®, may be attached to transparent tubing and a 23 gauge butterfly needle for injection. Insert the needle and draw the patient’s blood to the hub of the syringe so that a small air bubble separates the patient’s blood in the tubing from the fluorescein. With the room lights on, slowly inject the blood back into the vein while watching the skin over the needle tip. If the needle has extravasated, the patient’s blood will be seen to bulge the skin and the injection should be stopped before any fluorescein is injected. When assured that extravasation has not occurred, the room light may be turned off and the fluorescein injection completed. Luminescence usually appears in the retina and choroidal vessels in 7 to 14 seconds and can be observed by standard viewing equipment.
Reduction in dose from 5 mL to 2 mL of FLUORESCITE® Injection 10% may be appropriate in cases when a highly sensitive imaging system e.g., scanning laser ophthalmoscope is used.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
FLUORESCITE® Injection 10% is contraindicated in patients with known hypersensitivity to fluorescein sodium or any other ingredients in this product. Rare cases of death due to anaphylaxis have been reported. [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
Fluorescein sodium can induce serious intolerance reactions. These reactions of intolerance are always unpredictable but they are more frequent in patients who have previously experienced an adverse reaction after fluorescein injection (symptoms other than nausea and vomiting) or in patients with history of allergy such as food or drug induced urticaria, asthma, eczema, allergic rhinitis.
Detailed questioning of each patient is recommended before the angiography to evaluate any prior history of allergy.
-
5 WARNINGS AND PRECAUTIONS
5.1 Respiratory Reactions
Caution is to be exercised in patients with a history of allergy or bronchial asthma. An emergency tray should be available in the event of possible reaction to FLUORESCITE® Injection 10%.
If a potential allergy is suspected, an intradermal skin test may be performed prior to intravenous administration, i.e., 0.05 mL injected intradermally to be evaluated 30 to 60 minutes following injection. Given the sensitivity and specificity of skin testing, a negative skin test is not proof that a patient is not allergic to fluorescein.
5.2 Severe Local Tissue Damage
Care must be taken to avoid extravasation during injection as the high pH of fluorescein solution can result in severe local tissue damage. The following complications resulting from extravasation of fluorescein have been noted to occur: severe pain in the arm for several hours, sloughing of the skin, superficial phlebitis, subcutaneous granuloma, and toxic neuritis along the median curve in the antecubital area. When significant extravasation occurs, the injection should be discontinued and conservative measures to treat damaged tissue and to relieve pain should be implemented. [see Administration (2.3) and Adverse Reactions(6)].
-
6 ADVERSE REACTIONS
Skin and Urine Discoloration
The most common reaction is temporary yellowish discoloration of the skin and urine. Urine may attain a bright yellow color. Discoloration of the skin usually fades in 6 to 12 hours and usually fades in urine in 24 to 36 hours.Gastrointestinal Reactions
Nausea, vomiting, and gastrointestinal distress are common adverse events. A strong taste may develop after injection.
Hypersensitivity Reactions
Symptoms and signs of hypersensitivity have occurred. Generalized hives and itching, bronchospasm and anaphylaxis have been reported. Rare cases of death have been reported. [see Contraindications (4.1) and Warnings and Precautions (5.1)].
Cardiopulmonary Reactions
Cardiac arrest, basilar artery ischemia, severe shock and death may occur rarely.Neurologic Reactions
Headache may occur. Convulsions and syncope may rarely occur following injection.Thrombophlebitis
Thrombophlebitis at the injection site has been reported. Extravasation of the solution at the injection site causes intense pain at the site and a dull aching pain in the injected arm. [see Administration (2.3) and Warnings and Precautions (5.2)]. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Adequate animal reproduction studies have not been conducted with fluorescein sodium. It is also not known whether fluorescein sodium can cause fetal harm when administered to a pregnant woman. Fluorescein sodium should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
Fluorescein sodium injection has been demonstrated to be excreted in human milk for up to 4 days. Following fluorescein angiography, breast-feeding should therefore be discontinued for at least 4 days and the milk should be pumped off and discarded during this period.
-
11 DESCRIPTION
FLUORESCITE® (fluorescein injection, USP) 10% contains fluorescein sodium (equivalent to fluorescein 10% w/v). It is a sterile solution for use intravenously as a diagnostic aid. Its chemical name is spiro[isobenzofuran-1(3H ),9'-[9H]xanthene]-3-one, 3'6'-dihydroxy, disodium salt. The active ingredient is represented by the chemical structure:
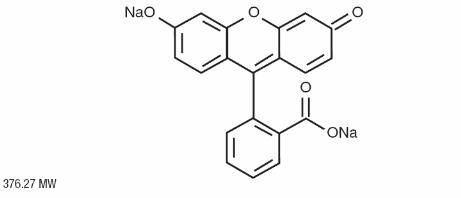
FLUORESCITE® (fluorescein injection, USP) 10% is supplied as a sterile, unpreserved, unit dose aqueous solution, that has a pH of 8.0 – 9.8 and an osmolality of 572-858 mOsm/kg.
Active ingredient: fluorescein sodium
Inactive Ingredients: Sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fluorescein sodium responds to electromagnetic radiation and light between the wavelengths of 465-490 nm and fluoresces, i.e., emits light at wavelengths of 520-530 nm. Thus, the hydrocarbon is excited by blue light and emits light that appears yellowish-green. Following intravenous injection of fluorescein sodium in an aqueous solution, the unbound fraction of the fluorescein can be excited with a blue light flash from a fundus camera as it circulates through the ocular vasculature, and the yellowish green fluorescence of the dye is captured by the camera. In the fundus, the fluorescence of the dye demarcates the retinal and/or choroidal vasculature under observation, distinguishing it from adjacent areas/structures.
12.3 Pharmacokinetics
Distribution:
Within 7 to 14 seconds after intravenous (IV) administration into antecubital vein, fluorescein usually appears in the central artery of the eye. Within a few minutes of IV administration of fluorescein sodium, a yellowish discoloration of the skin occurs, which begins to fade after 6 to 12 hours of dosing. Various estimates of volume of distribution indicate that fluorescein distributes well into interstitial space (0.5 L/kg).
Metabolism:
Fluorescein undergoes rapid metabolism to fluorescein monoglucuronide. After IV administration of fluorescein sodium (14 mg/kg) to 7 healthy subjects, approximately 80% of fluorescein in plasma was converted to glucuronide conjugate after a period of 1 hour post dose, indicating relatively rapid conjugation.
Excretion:
Fluorescein and its metabolites are mainly eliminated via renal excretion. After IV administration, the urine remains slightly fluorescent for 24 to 36 hours. A renal clearance of 1.75 mL/min/kg and a hepatic clearance (due to conjugation) of 1.50 mL/min/kg have been estimated. The systemic clearance of fluorescein was essentially complete by 48 to 72 hours after administration of 500 mg fluorescein.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
FLUORESCITE®(fluorescein injection, USP) 10% is supplied in a single use 5 mL glass vial with a gray FluroTec coated chlorobutyl stopper and purple flip-off aluminum seal. The vial stopper is not made with natural rubber latex. The vial contains a sterile, red-orange solution of fluorescein sodium.
NDC 0065-0092-65
-
17 PATIENT COUNSELING INFORMATION
After administration of fluorescein sodium, skin will attain a temporary yellowish discoloration. Urine attains a bright yellow color. Discoloration of the skin usually fades in 6 to 12 hours and usually fades in urine in 24 to 36 hours. [see Adverse Reactions (6)]
Distributed By:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
© 2006, 2016 Novartis
Revised: 2/2016
9014068-0117
- PATIENT PACKAGE INSERT
-
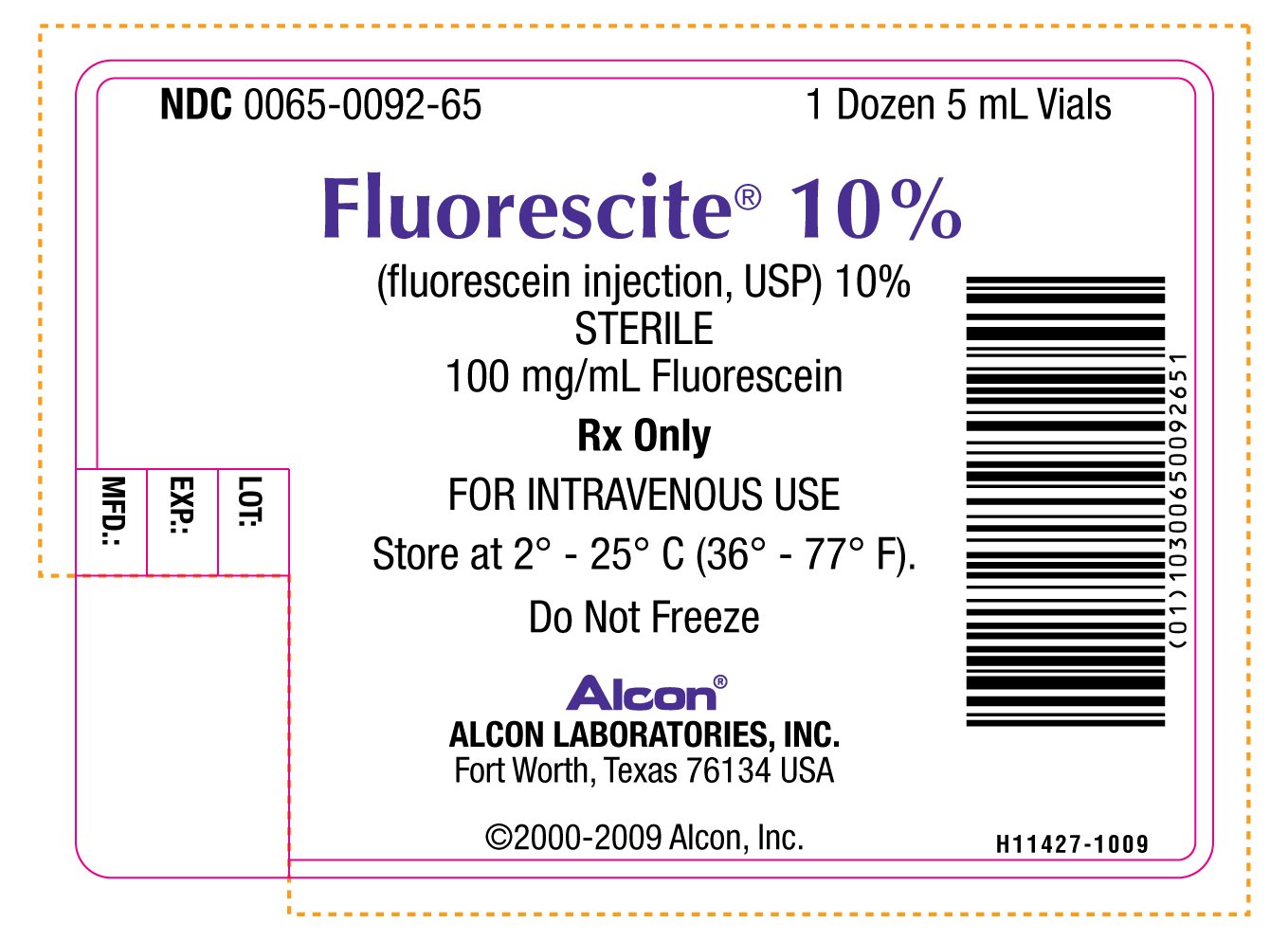
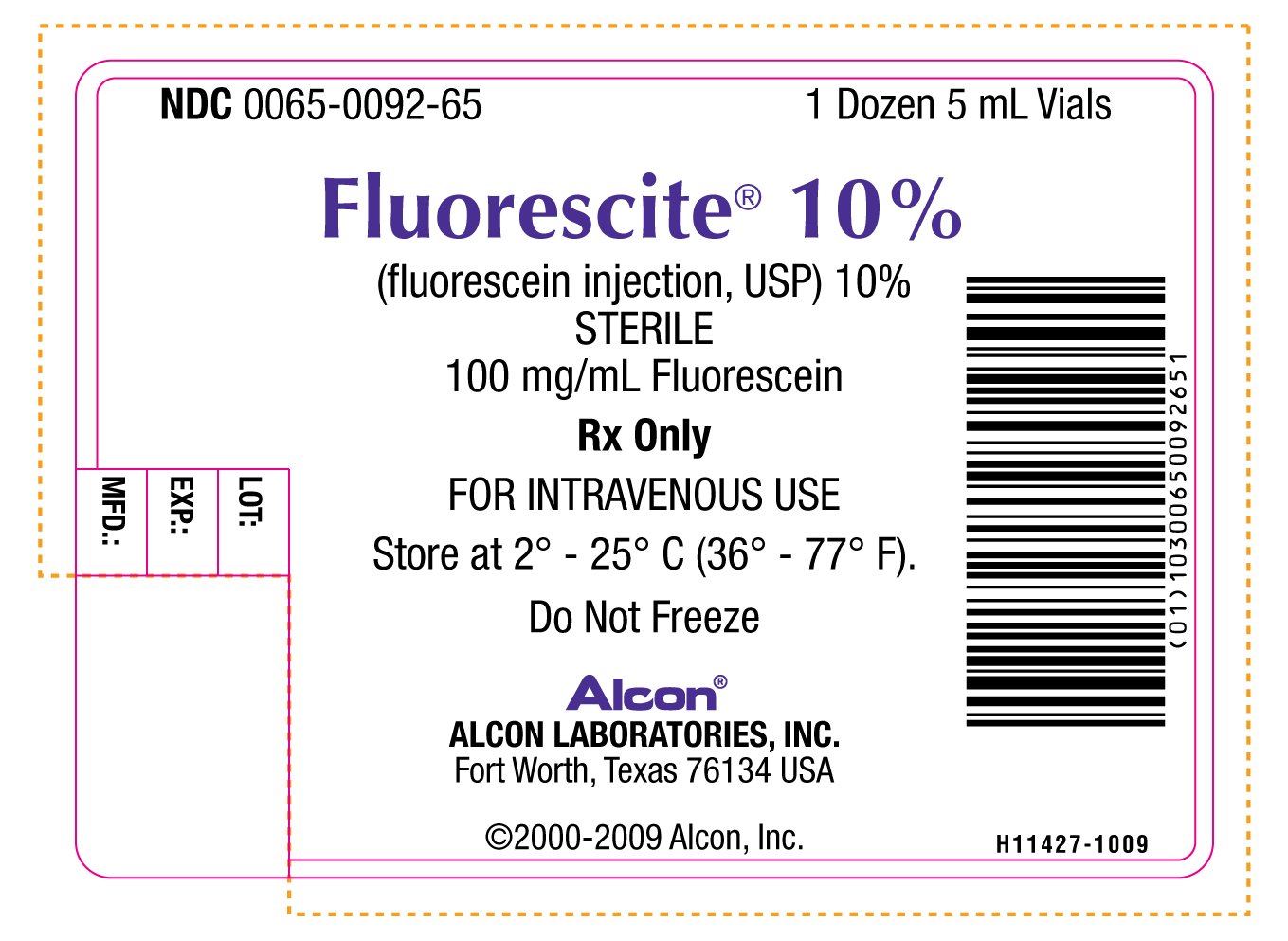
PRINCIPAL DISPLAY PANEL
NDC 0065-0092-65 1 Dozen 5 mL Vials
Florescite® 10%
(fluorescein injection, USP) 10%
STERILE
100 mg/mL Fluorescein
Rx Only
FOR INTRAVENOUS USE
Store at 2° - 25° C (36° - 77° F)
Do Not Freeze
Alcon®
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
©2000-2009 Alcon, Inc.
H11427-1009
LOT:
EXP.:
MFD.:
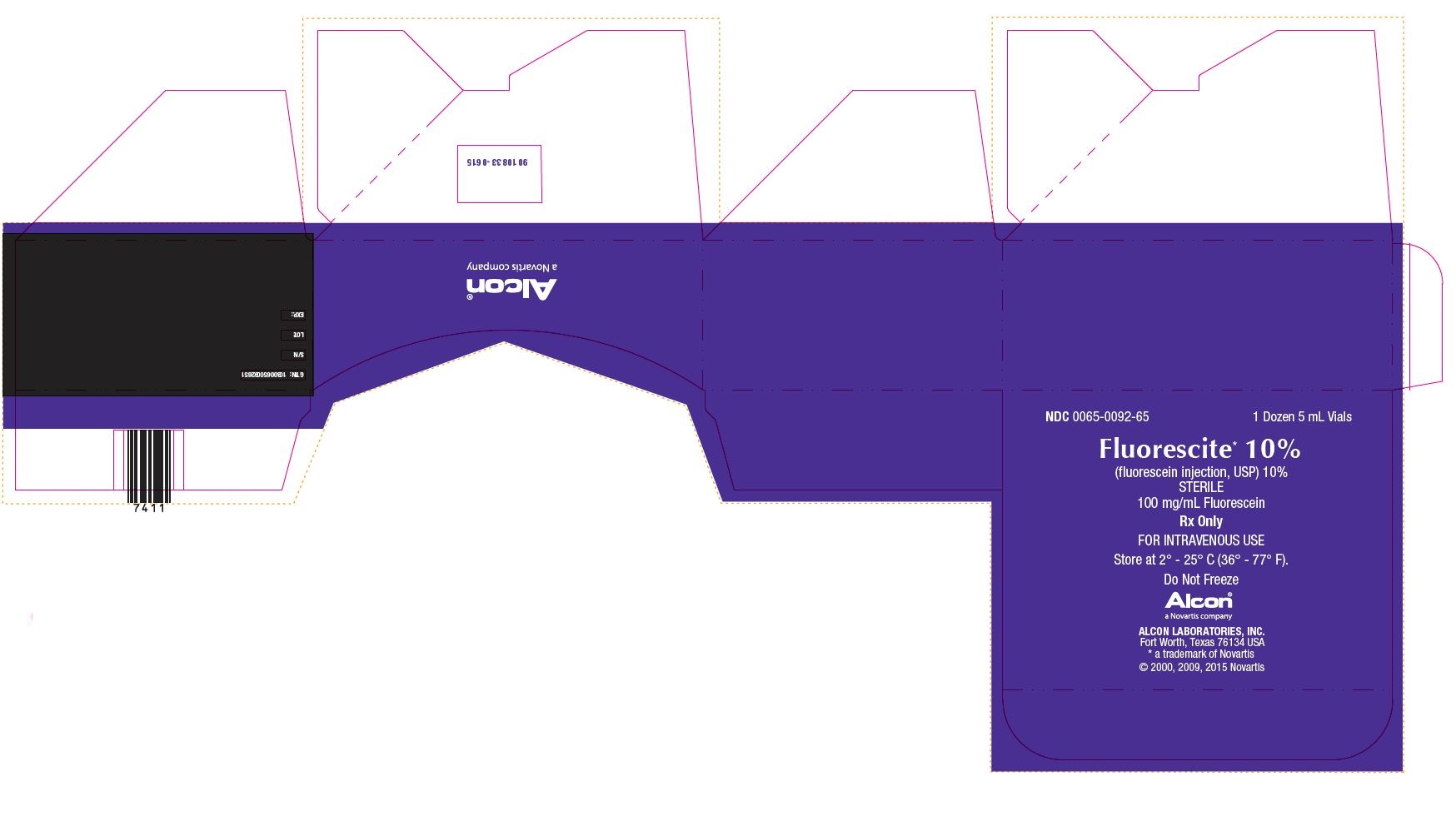
NDC 0065-0092-65 1 Dozen 5 mL Vials
Florescite* 10%
(fluorescein injection, USP) 10%
STERILE
100 mg/mL Fluorescein
Rx Only
FOR INTRAVENOUS USE
Store at 2° - 25° C (36° - 77° F)
Do Not Freeze
Alcon®
a Novartis company
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
* a trademark of Norvatis
©2000, 2009, 2015 Novartis
GTIN: 10300650092651
S/N:
LOT:
EXP.:
9010833-0615
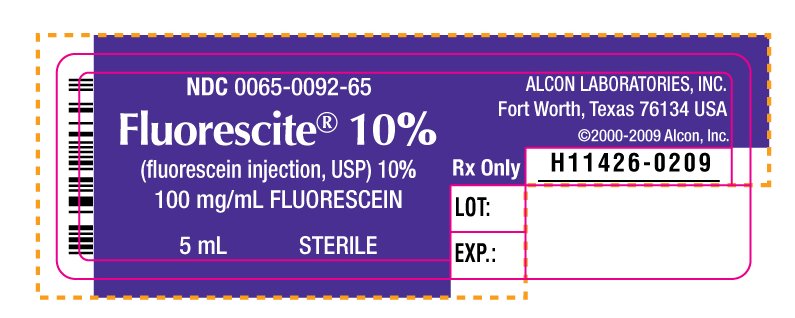
NDC 0065-0092-65
Fluorescite® 10%
(fluorescein injection, USP) 10%
100 mg/mL FLUORESCEIN
5 mL STERILE
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
©2000-2009 Alcon, Inc.
Rx Only
H11426-0209
LOT:
EXP.:
-
INGREDIENTS AND APPEARANCE
FLUORESCITE
fluorescein sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0065-0092 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUORESCEIN SODIUM (UNII: 93X55PE38X) (FLUORESCEIN - UNII:TPY09G7XIR) FLUORESCEIN 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0092-05 12 in 1 CARTON 09/15/1972 10/31/2013 1 5 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:0065-0092-65 12 in 1 CARTON 09/15/1972 2 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021980 09/15/1972 Labeler - Alcon, Inc. (008018525) Registrant - Alcon Research LLC (007672236) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0092) Establishment Name Address ID/FEI Business Operations INTERNATIONAL MEDICATION SYSTEMS, LIMITED 055750020 MANUFACTURE(0065-0092)