Label: DURAFLEX- ascorbic acid, calcium, glucosamine sulfate, chondroitin sulfate, msm and cat claw. tablet
- NHRIC Code(s): 43063-965-60, 43063-965-98
- Packager: PD-Rx Pharmaceuticals, Inc.
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated July 16, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HEALTH CLAIM:
-
DESCRIPTION:
duraflex is formulated to help support healthy, mobile joint function and connective tissue, and therefore, support the comfortable freedom of movement often lost as we age. When taken daily over time, duraflex helps protect cartilage from occasional overexertion and physical activity as it provides the nourishment your body needs to support cartilage, lubricate, and strengthen your joints. duraflex has key dietary ingredients, including: Glucosamine; Chondroitin; Methylsulfonylmethane (MSM). With daily use, benefits may be felt in as little as four weels.†
† The above statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
-
WARNING AND PRECAUTIONS
Pregnant or lactating women should consult a doctor using any product. Avoid product if you have allergies to shellfish. Consult doctor before use if you have, or have had, thyroid or prostate disorders, diabetes, hypoglycemia, asthma, or if you are on a sodium restricted diet or if you are taking ANY MEDICATIONS OR REMEDIES such as blood thinners or daily aspirin. Discontinue use and consult your doctor before use if any adverse reactions occur, such as gastointestinal discomfort, allergic reaction, any skin irritation, fatigue, dizziness, pain, hair loss, irregular heartbeat, or changes in blood pressure or mood. NOT INTENDED FOR USE BY PERSONS UNDER THE AGE OF 18.
WARNING: If you are pregnant, nursing a baby, recovering from recent surgery or have a heart or circulatory condition, consult a physician before using this product.
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED HEALTH CLAIM:
- STORAGE AND HANDLING:
- PACKAGE LABEL:
-
INGREDIENTS AND APPEARANCE
DURAFLEX
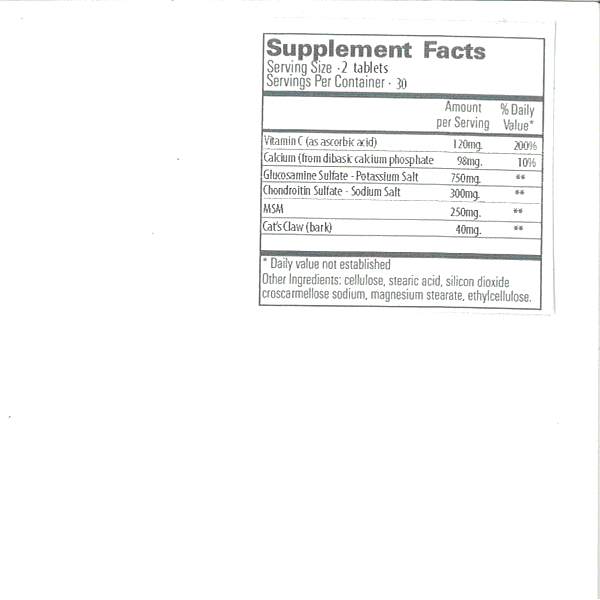
ascorbic acid, calcium, glucosamine sulfate, chondroitin sulfate, msm and cat claw. tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:43063-965 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 60 mg DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) (CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS - UNII:L11K75P92J) DIBASIC CALCIUM PHOSPHATE DIHYDRATE 49 mg CHONDROITIN SULFATE SODIUM (BOVINE) (UNII: 8QTV3DTT8W) (CHONDROITIN SULFATE (BOVINE) - UNII:6IC1M3OG5Z) CHONDROITIN SULFATE (BOVINE) 150 mg DIMETHYL SULFOXIDE (UNII: YOW8V9698H) (DIMETHYL SULFOXIDE - UNII:YOW8V9698H) DIMETHYL SULFOXIDE 125 mg CAT'S CLAW (UNII: 9060PRM18Q) (CAT'S CLAW - UNII:9060PRM18Q) CAT'S CLAW 175 mg Inactive Ingredients Ingredient Name Strength GLUCOSAMINE SULFATE POTASSIUM CHLORIDE (UNII: 15VQ11I66N) 375 mg POWDERED CELLULOSE (UNII: SMD1X3XO9M) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:43063-965-60 60 in 1 BOTTLE, PLASTIC 2 NHRIC:43063-965-98 120 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 07/16/2021 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 size (solid drugs) 21 mm shape Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) Establishment Name Address ID/FEI Business Operations PD-Rx Pharmaceuticals, Inc. 156893695 repack(43063-965)