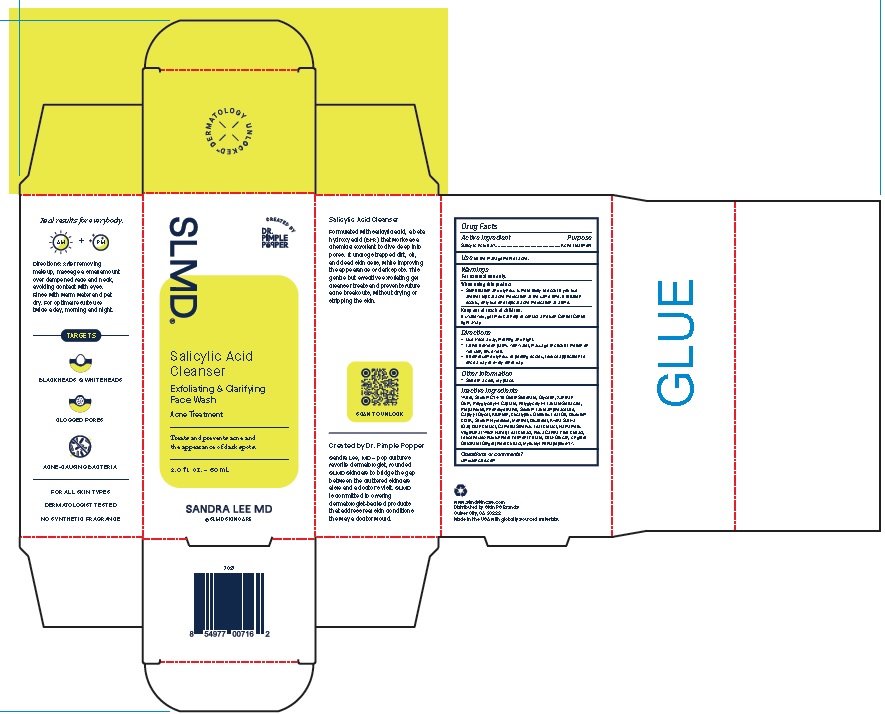
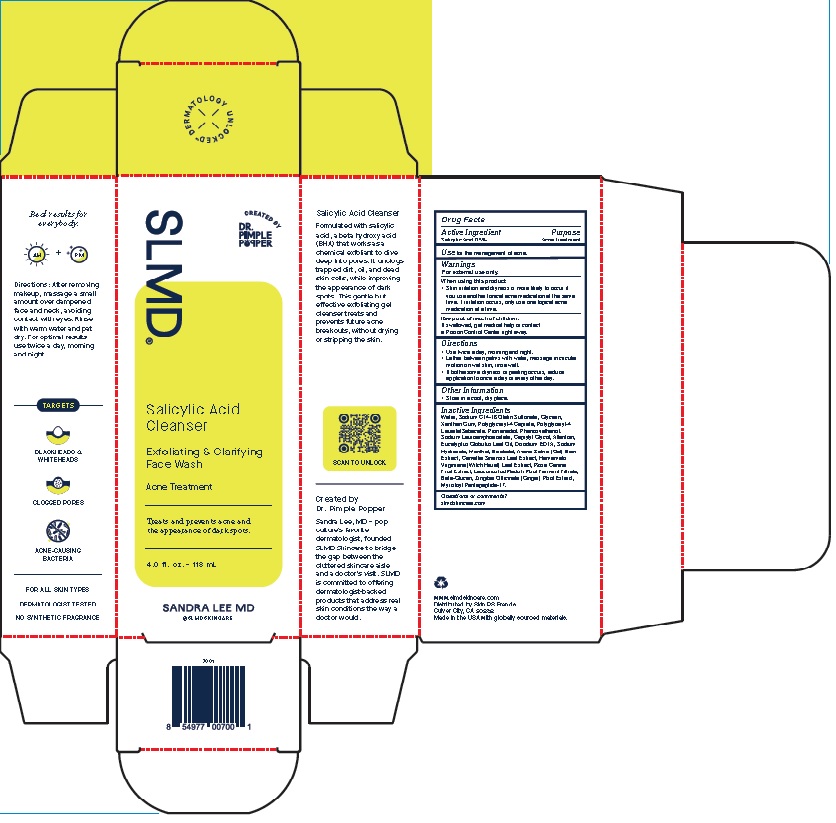
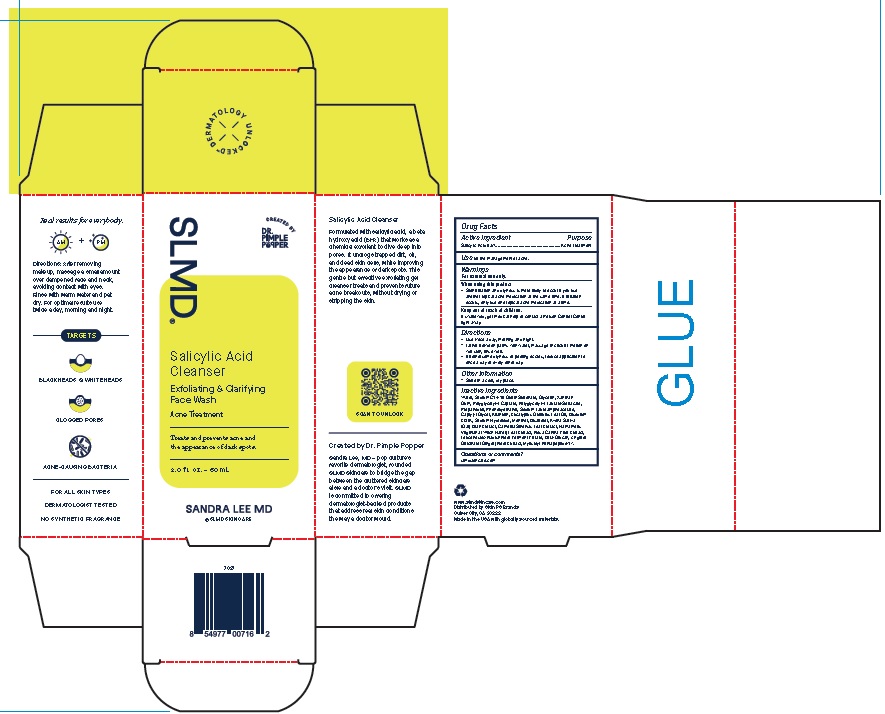
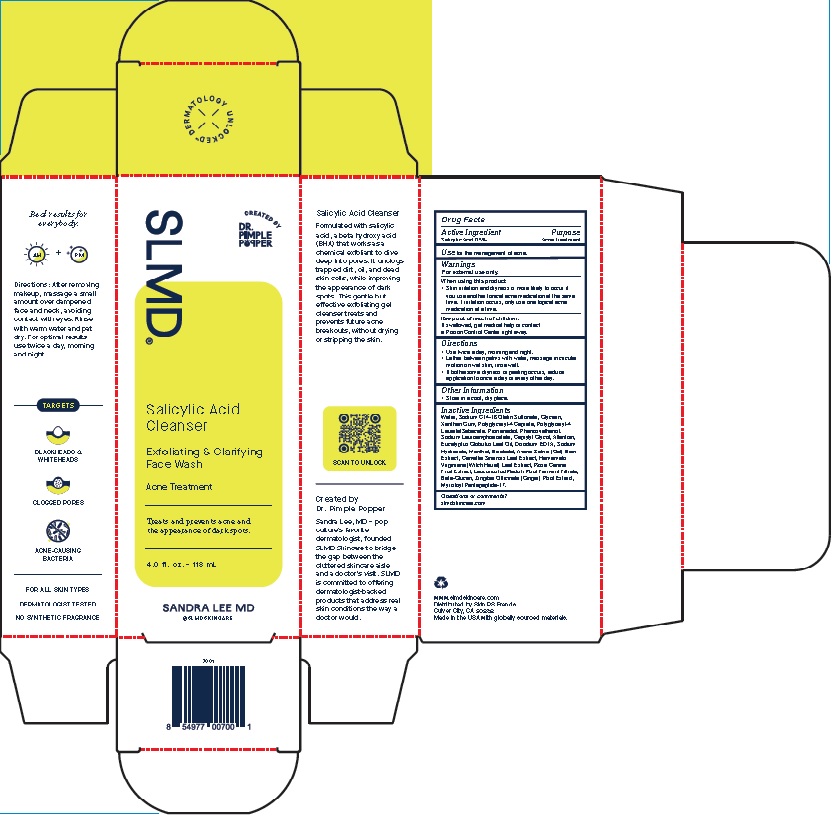
Label: SALICYLIC ACID CLEANSER- exfoliating and clarifying face wash gel
- NDC Code(s): 54111-171-02, 54111-171-04
- Packager: Bentley Laboratories, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive Ingredients
Water, Sodium C14-16 Olefin Sulfonate, Glycerin, Xanthan
Gum, Polyglyceryl-4 Caprate, Polyglyceryl-4 Laurate/Sebacate,
Propanediol, Phenoxyethanol, Sodium Lauroamphoacetate,
Caprylyl Glycol, Allantoin, Eucalyptus Globulus Leaf Oil, Disodium
EDTA, Sodium Hydroxide, Menthol, Bisabolol, Avena Sativa
(Oat) Bran Extract, Camellia Sinensis Leaf Extract, Hamamelis
Virginiana (Witch Hazel) Leaf Extract, Rosa Canina Fruit Extract,
Leuconostoc/Radish Root Ferment Filtrate, Beta-Glucan, Zingiber
Officinale (Ginger) Root Extract, Myristoyl Pentapeptide-17. - QUESTIONS
-
Product Package for Salicylic Acid Cleanser
SLMD®
Salicylic Acid
Cleanser
Exfoliating & Clarifying
Face WashAcne Treatment
Treats and prevents acne and
the appearance of dark spots.2.0 f l. oz. – 60 mL
SANDRA LEE MD
w w w.slmdskincare.com
Distributed by Skin PS Brands
Culver City, CA 90232Made in the USA with globally sourced materials.
2 Oz.

4 Oz.

res
-
INGREDIENTS AND APPEARANCE
SALICYLIC ACID CLEANSER
exfoliating and clarifying face wash gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54111-171 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) POLYGLYCERYL-4 CAPRATE (UNII: 3N873UN885) PROPANEDIOL (UNII: 5965N8W85T) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM LAUROAMPHOACETATE (UNII: SLK428451L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALLANTOIN (UNII: 344S277G0Z) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SODIUM HYDROXIDE (UNII: 55X04QC32I) MENTHOL (UNII: L7T10EIP3A) LEVOMENOL (UNII: 24WE03BX2T) OAT BRAN (UNII: KQX236OK4U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HAMAMELIS VIRGINIANA LEAF (UNII: T07U1161SV) ROSA CANINA FRUIT (UNII: 3TNW8D08V3) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) TRANSFORMING GROWTH FACTOR BETA RECEPTOR TYPE 3 (UNII: 18YWT2KYS8) GINGER (UNII: C5529G5JPQ) MYRISTOYL PENTAPEPTIDE-4 (UNII: PMA59A699X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54111-171-02 1 in 1 CARTON 07/01/2023 1 60 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:54111-171-04 1 in 1 CARTON 07/01/2023 2 118 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 07/01/2023 Labeler - Bentley Laboratories, LLC (068351753) Registrant - Skin PS Brands (081085221)