Label: WHITE PETROLATUM ointment
- NDC Code(s): 0404-0169-50
- Packager: Henry Schein
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use(s)
- Warnings
- Directions
- Other Information
- Label
-
INGREDIENTS AND APPEARANCE
WHITE PETROLATUM
white petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0404-0169 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WHITE PETROLATUM (UNII: B6E5W8RQJ4) (WHITE PETROLATUM - UNII:B6E5W8RQJ4) WHITE PETROLATUM 1 g in 1 g Product Characteristics Color Score Shape FREEFORM Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0404-0169-50 864 in 1 CASE 11/17/2022 1 144 in 1 BOX 1 5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/17/2022 Labeler - Henry Schein (012430880)


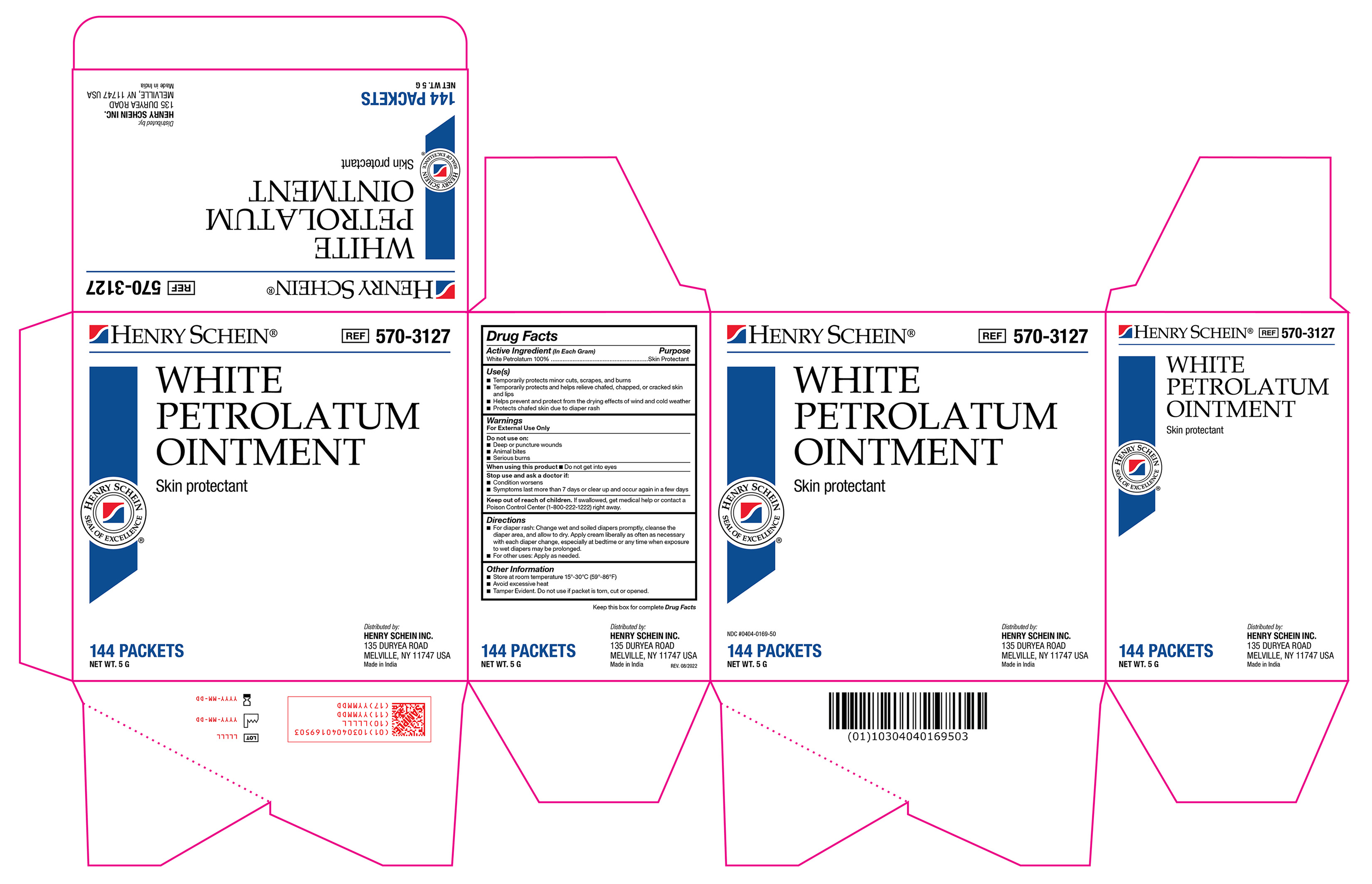 570-3127 Petrolatum Ointment
570-3127 Petrolatum Ointment