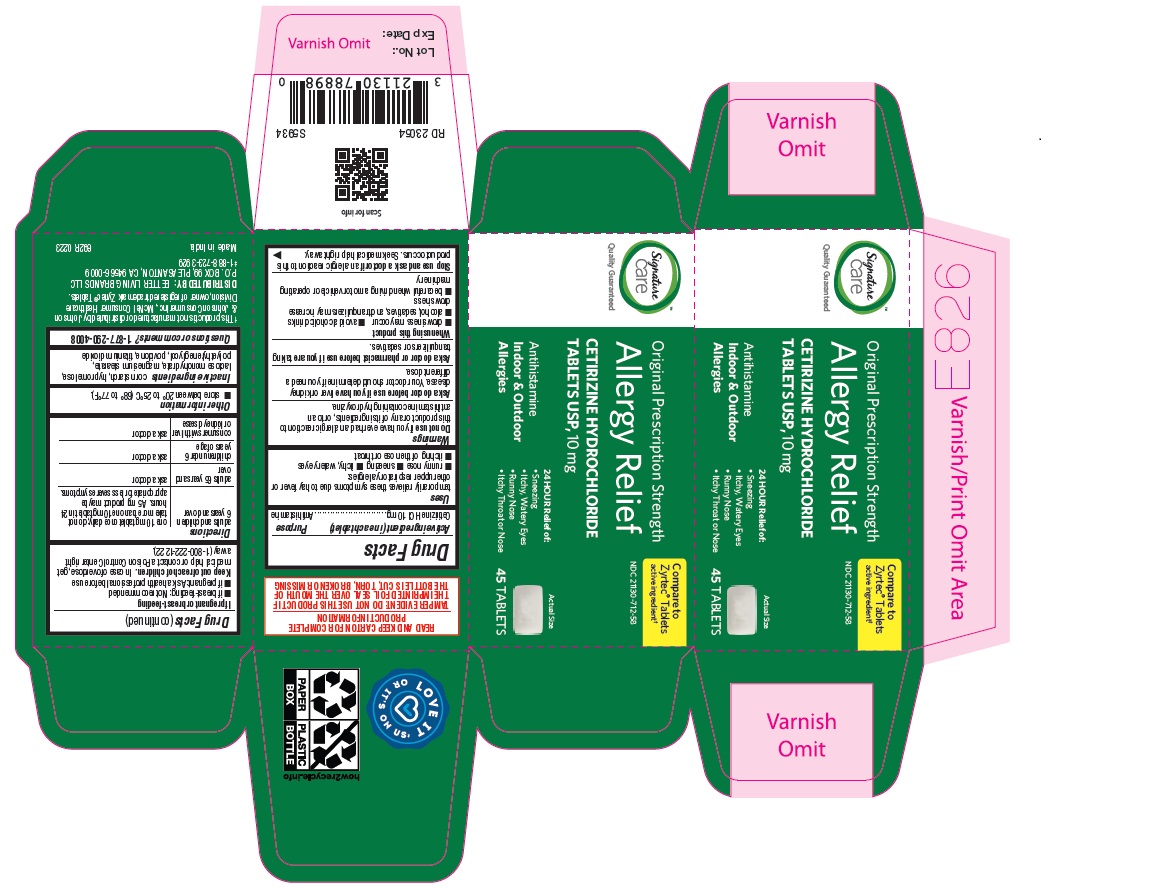
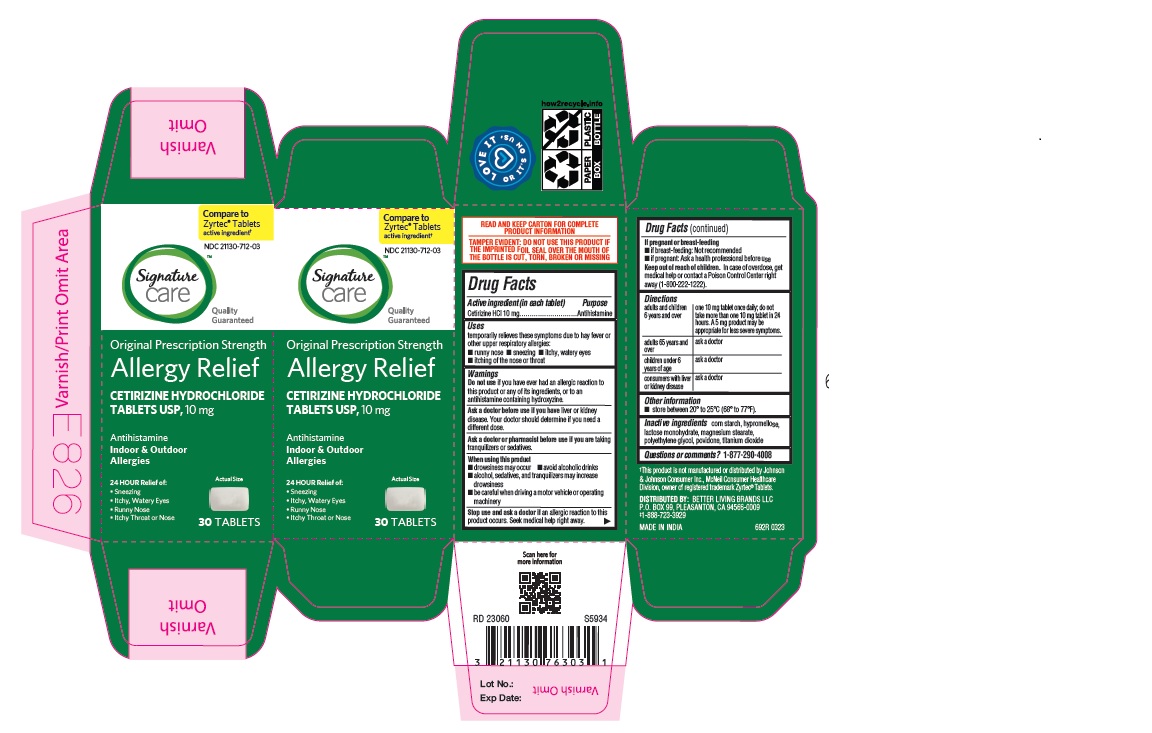
Label: ALLERGY RELIEF- cetirizine hydrochloride tablet
-
NDC Code(s):
21130-712-03,
21130-712-09,
21130-712-12,
21130-712-46, view more21130-712-58, 21130-712-81
- Packager: BETTER LIVING BRANDS, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
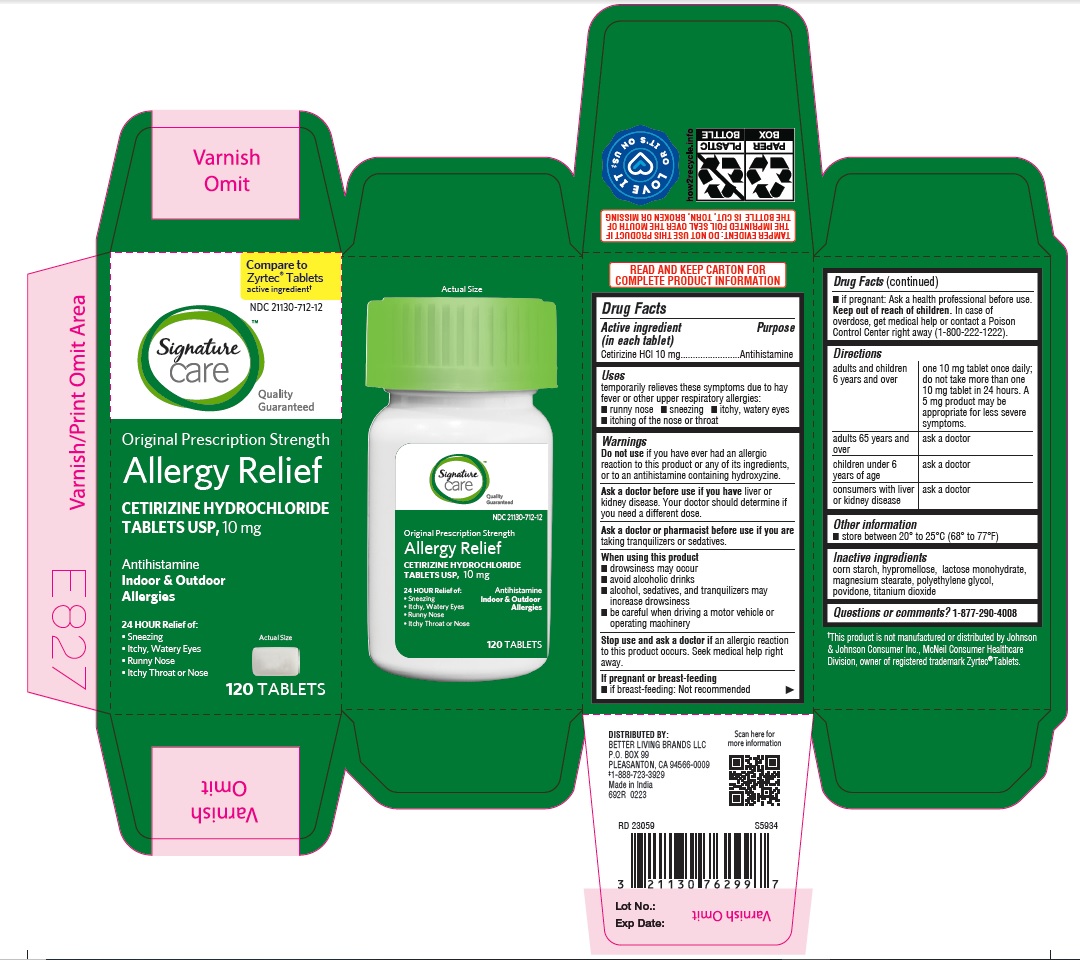
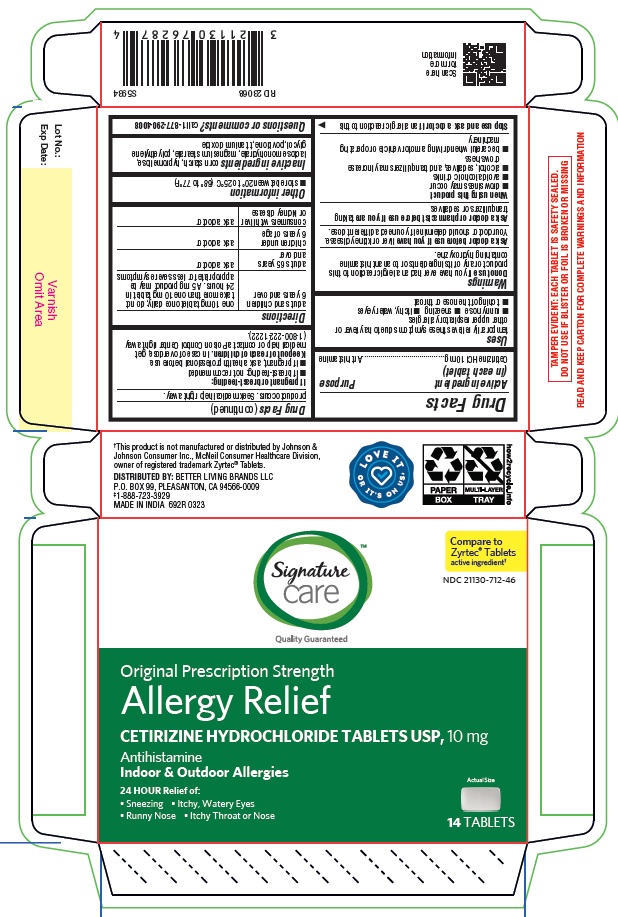
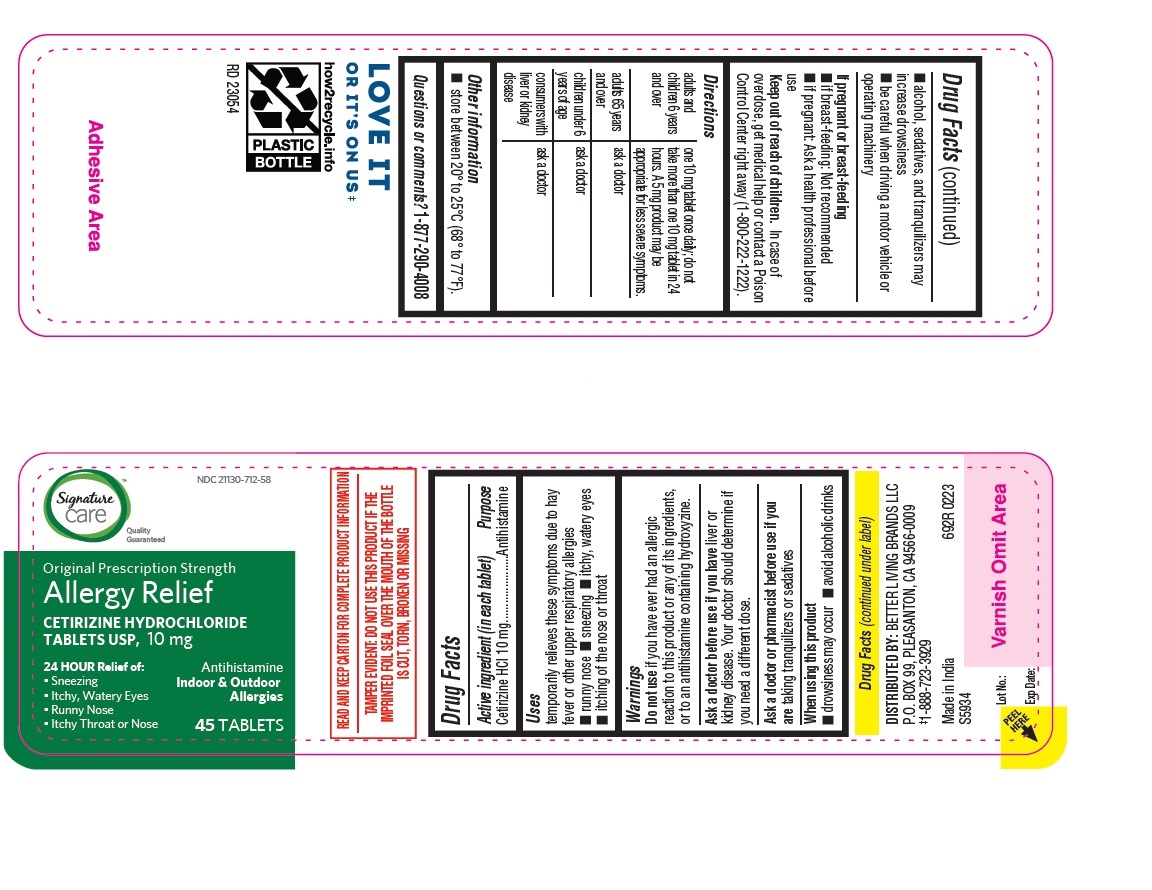
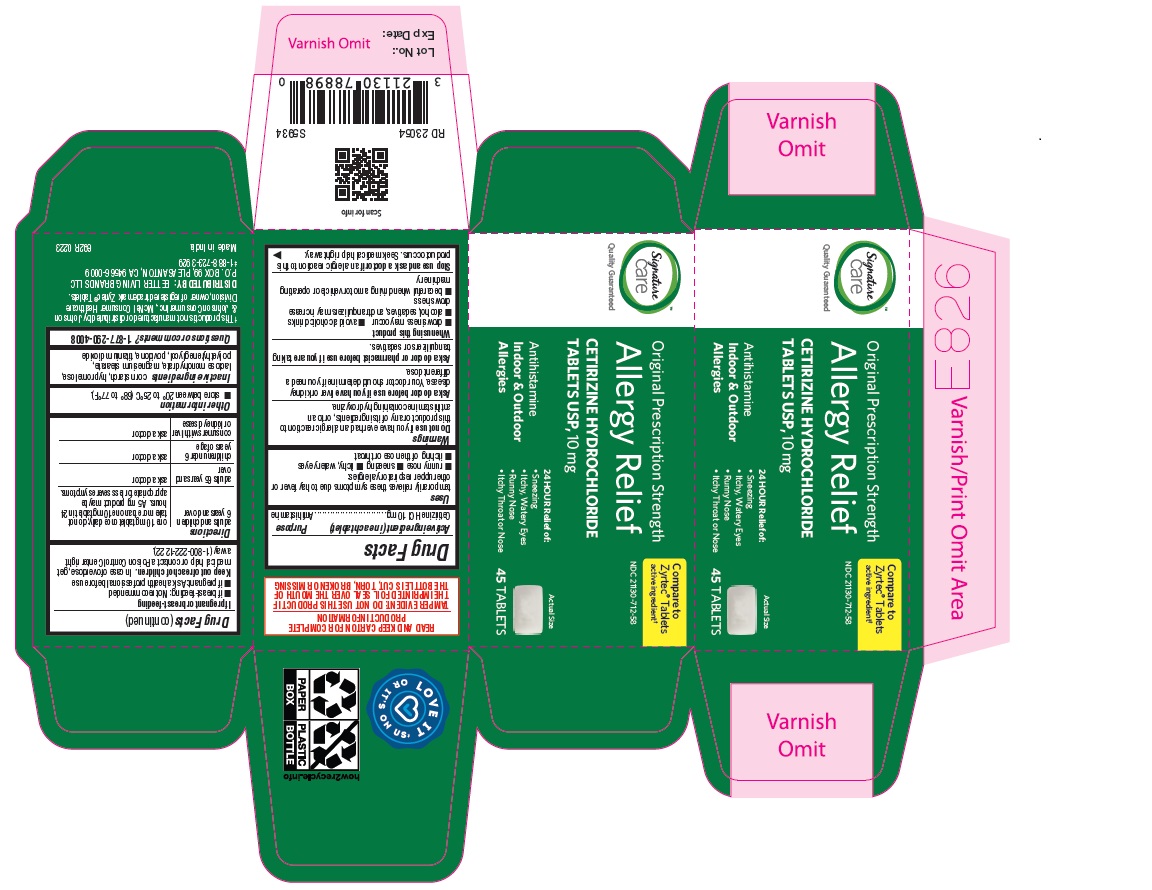
adults and children 6 years and over one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms. adults 65 years and over ask a doctor
children under 6 years of age ask a doctor
consumers with liver or kidney disease ask a doctor
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
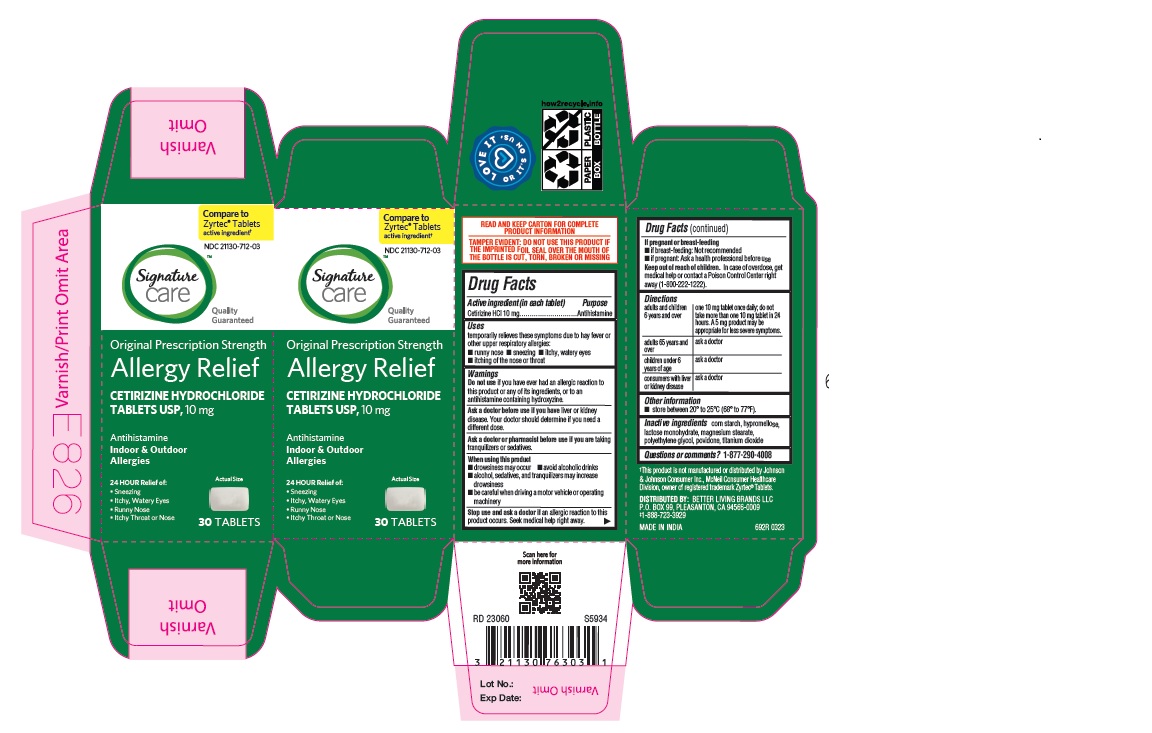
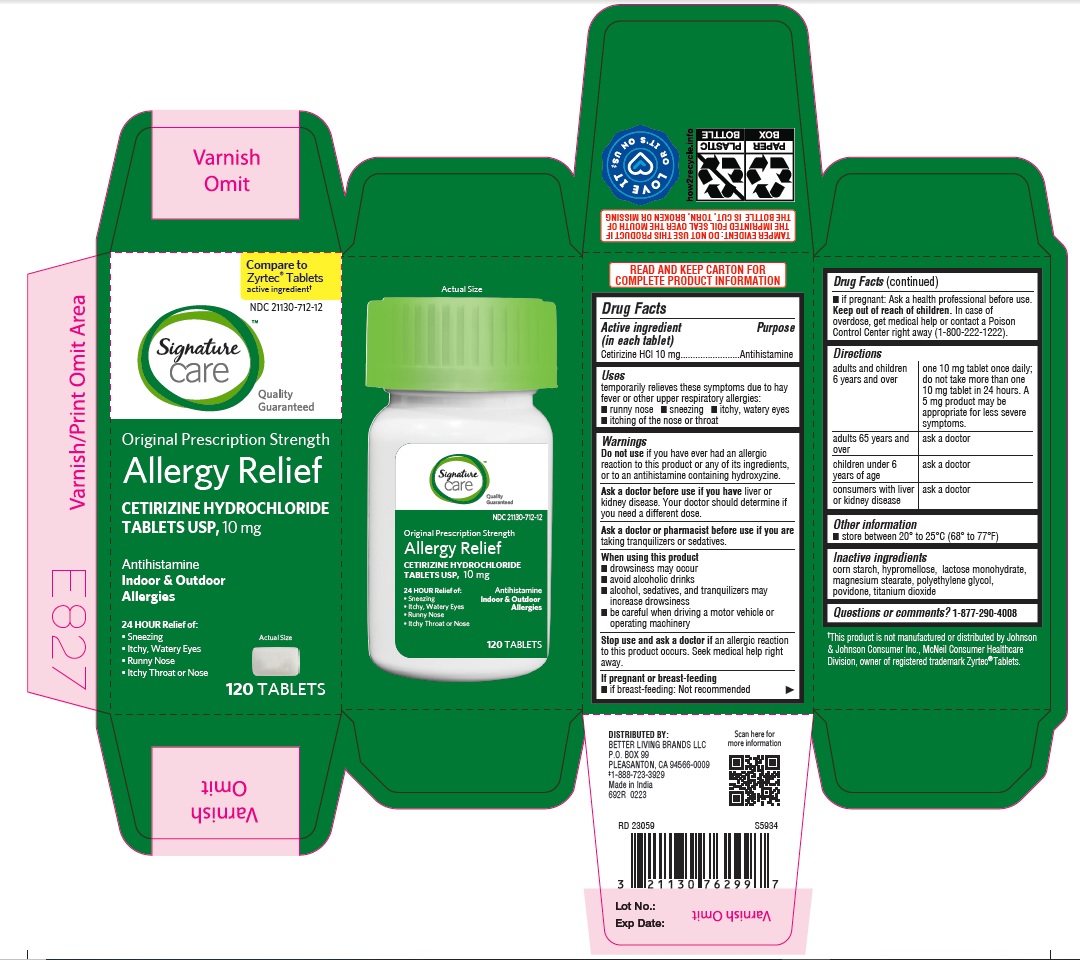
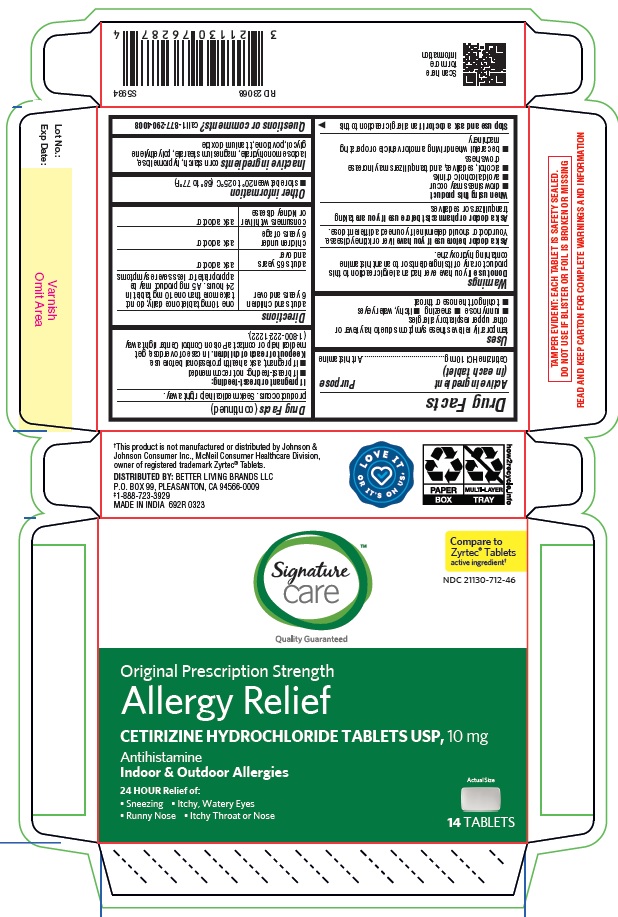
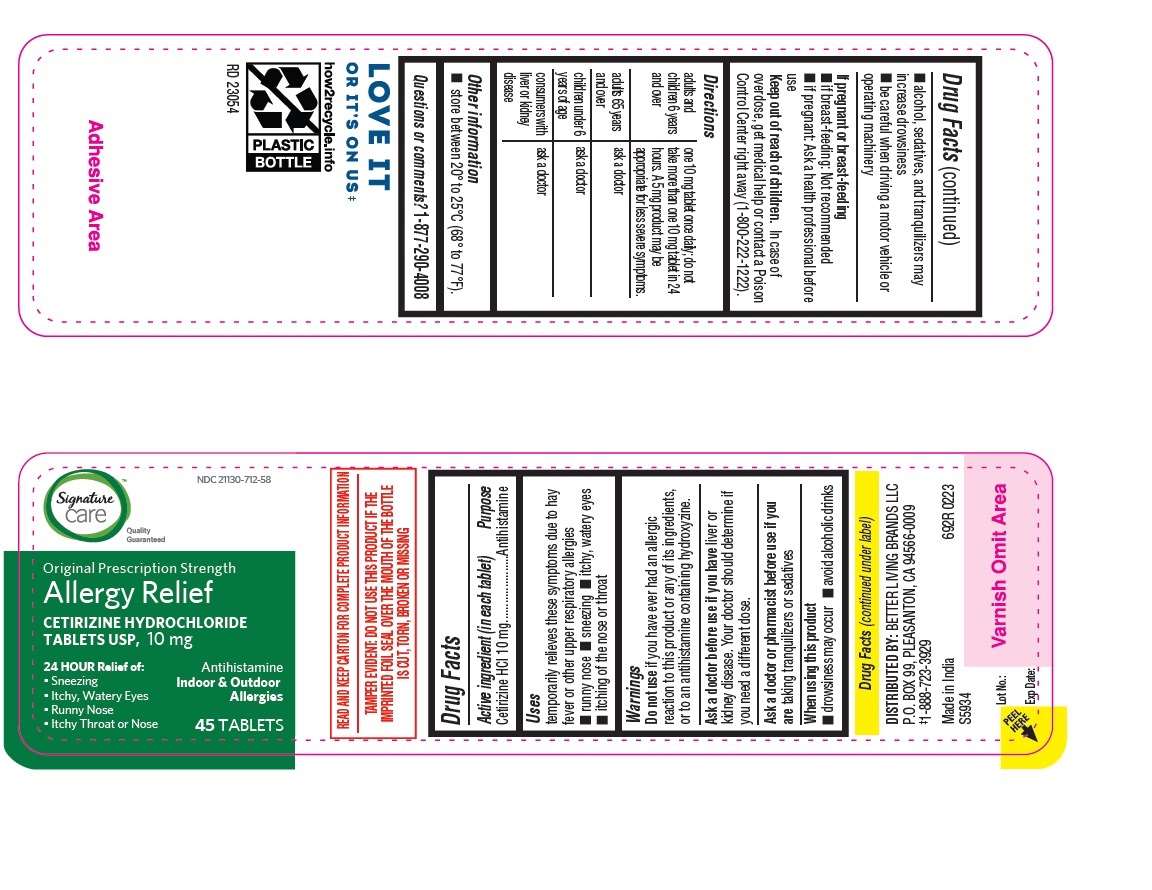
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
cetirizine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-712 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (White to off white) Score no score Shape RECTANGLE (Rounded-off, rectangular shaped tablet) Size 9mm Flavor Imprint Code J;220 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-712-58 1 in 1 CARTON 05/31/2023 1 45 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:21130-712-03 1 in 1 CARTON 05/31/2023 2 30 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:21130-712-46 1 in 1 CARTON 06/26/2023 3 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:21130-712-81 2 in 1 CARTON 06/23/2023 4 NDC:21130-712-09 90 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:21130-712-12 1 in 1 CARTON 08/30/2023 5 120 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078933 05/31/2023 Labeler - BETTER LIVING BRANDS, LLC (009137209) Registrant - TIME CAP LABORATORIES, INC. (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 manufacture(21130-712)