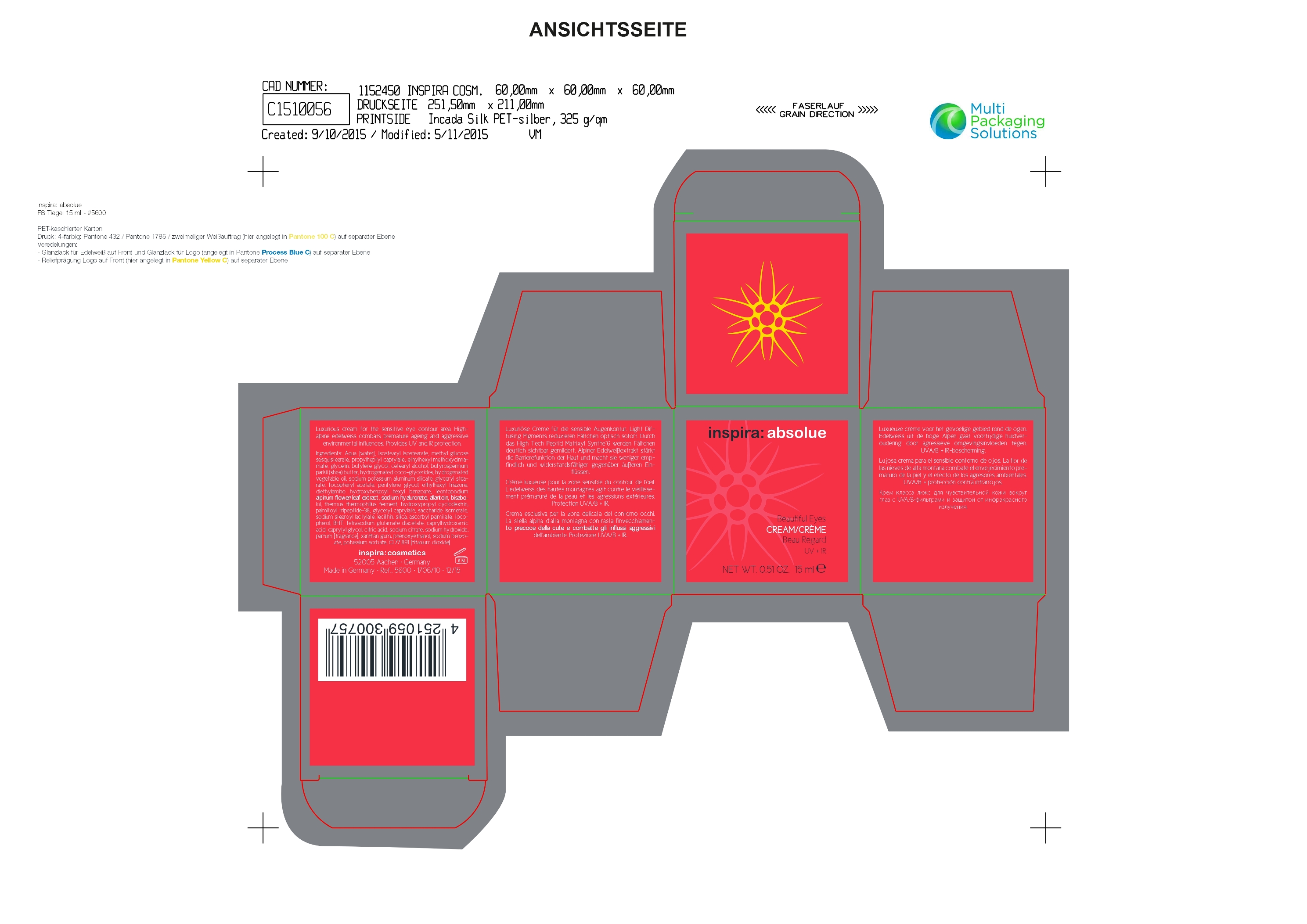
Label: ETHYLHEXYL METHOXYCINNAMATE CREAM- beautiful eyes cream cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70493-560-01, 70493-560-03 - Packager: inspira: cosmetics GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 16, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Aqua [water], isostearyl isostearate, methyl glucose,sesquistearate, propylheptyl caprylate,glycerin, butylene glycol, cetearyl alcohol, butyrospermumparkii (shea) butter, hydrogenated coco-glycerides, sodium potassium aluminum silicate, glyceryl stearate,tocopheryl acetate, pentylene glycol, ethylhexyl triazone, diethylamino hydroxybenzoyl hexyl benzoate, leontopodium
alpinum flower/leaf extract, sodium hyaluronate, allantoin, bisabolol, thermus thermophillus ferment, hydroxypropyl cyclodextrin,
palmitoyl tripeptide-38, glyceryl caprylate, saccharide isomerate, sodium stearoyl lactylate, lecithin, silica, ascorbyl palmitate, tocopherol, BHT, tetrasodium glutamate diacetate, caprylhydroxamic acid, caprylyl glycol, citric acid, sodium citrate, sodium hydroxide, xanthan gum, phenoxyethanol, sodium benzoate, potassium sorbate, CI 77 891 [titanium dioxide] - Beautiful Eyes Cream
-
INGREDIENTS AND APPEARANCE
ETHYLHEXYL METHOXYCINNAMATE CREAM
beautiful eyes cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70493-560 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.6 g in 15 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL BETADEX (UNII: 1I96OHX6EK) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) ISOSTEARYL ISOSTEARATE (UNII: IV0Z586Z4Y) METHYL GLUCOSE SESQUISTEARATE (UNII: V1YW10H14D) PROPYLHEPTYL CAPRYLATE (UNII: 991Z19V2OD) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SHEA BUTTER (UNII: K49155WL9Y) HYDROGENATED COCO-GLYCERIDES (UNII: XDD37N2GPR) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) ETHYLHEXYL TRIAZONE (UNII: XQN8R9SAK4) DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE (UNII: ANQ870JD20) LEONTOPODIUM ALPINUM FLOWER (UNII: MWN6IZU3XM) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALLANTOIN (UNII: 344S277G0Z) LEVOMENOL (UNII: 24WE03BX2T) THERMUS THERMOPHILUS LYSATE (UNII: 775R692494) PALMITOYL LYSYLDIOXYMETHIONYLLYSINE (UNII: T7A529FB8O) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) SACCHARIDE ISOMERATE (UNII: W8K377W98I) SODIUM STEAROYL LACTYLATE (UNII: IN99IT31LN) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70493-560-03 1 in 1 BOX 02/01/2016 1 NDC:70493-560-01 15 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/01/2016 Labeler - inspira: cosmetics GmbH (329455898) Registrant - inspira: cosmetics GmbH (329455898) Establishment Name Address ID/FEI Business Operations inspira: cosmetics GmbH 329455898 manufacture(70493-560)