Label: PLAQUENIL- hydroxychloroquine sulfate tablet
-
NDC Code(s):
71610-473-04,
71610-473-30,
71610-473-53,
71610-473-74, view more71610-473-80
- Packager: Aphena Pharma Solutions - Tennessee, LLC
- This is a repackaged label.
- Source NDC Code(s): 59212-562
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PLAQUENIL® safely and effectively. See full prescribing information for PLAQUENIL.
PLAQUENIL (hydroxychloroquine sulfate) tablets, for oral use
Initial U.S. Approval: 1955INDICATIONS AND USAGE
PLAQUENIL is an antimalarial and antirheumatic indicated for the:
• Treatment of uncomplicated malaria due to Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax in adult and pediatric patients. (1.1)
• Prophylaxis of malaria in geographic areas where chloroquine resistance is not reported in adult and pediatric patients (1.1)
• Treatment of rheumatoid arthritis in adults. (1.2)
• Treatment of systemic lupus erythematosus in adults. (1.3)
• Treatment of chronic discoid lupus erythematosus in adults. (1.4)
Limitations of Use (1.1):
PLAQUENIL is not recommended for the:
• Treatment of complicated malaria.
• Treatment of chloroquine or hydroxychloroquine-resistant strains of Plasmodium species.
• Treatment of malaria acquired in geographic areas where chloroquine resistance occurs or when the Plasmodium species has not been identified.
• Prophylaxis of malaria in geographic areas where chloroquine resistance occurs.
• Prevention of relapses of P. vivax or P. ovale because it is not active against the hypnozoite liver stage forms of these parasites. For radical cure of P. vivax and P. ovale infections, concomitant therapy with an 8-aminoquinoline drug is necessary.DOSAGE AND ADMINISTRATION
Malaria in Adult and Pediatric Patients ( 2.2) :
- Prophylaxis: Begin weekly doses 2 weeks prior to travel to the endemic area, continue weekly doses while in the endemic area, and continue the weekly doses for 4 weeks after leaving the endemic area:
-Adults: 400 mg once a week
-Pediatric patients ≥ 31 kg: 6.5 mg/kg up to 400 mg, once a week - Treatment of Uncomplicated Malaria: See Full Prescribing Information (FPI) for complete dosing information.
Rheumatoid Arthritis in Adults ( 2.3) :
- Initial dosage: 400 mg to 600 mg daily
- Chronic dosage: 200 mg once daily or 400 mg once daily (or in two divided doses)
Systemic Lupus Erythematosus in Adults ( 2.4):
- 200 mg once daily or 400 mg once daily (or in two divided doses)
Chronic Discoid Lupus Erythematosus in Adults ( 2.5):
- 200 mg once daily or 400 mg once daily (or in two divided doses)
DOSAGE FORMS AND STRENGTHS
Tablets: 200 mg of hydroxychloroquine sulfate (3)
CONTRAINDICATIONS
• Patients with hypersensitivity to 4-aminoquinoline compounds ( 4)
WARNINGS AND PRECAUTIONS
• Cardiomyopathy and Ventricular Arrhythmias: Fatal or life-threatening cardiomyopathy and ventricular arrhythmias were reported. (5.1)
• Retinal Toxicity: Irreversible retinal damage is related to cumulative dosage and treatment duration. Baseline retinal exam and exams during treatment are recommended. (5.2)
• Serious Skin Reactions: Stevens Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, acute generalized exanthematous pustulosis have been reported. (5.3)
• Worsening of Psoriasis and Porphyria: Avoid in patients with psoriasis or porphyria. (5.4)
• Hematologic Toxicity: Discontinue if myelosuppression occurs. (5.5)• Renal Toxicity:Consider phospholipidosis as a possible cause of renal injury in patients with underlying connective tissue disorders. Discontinue PLAQUENIL if renal toxicity is suspected or demonstrated by tissue biopsy ( 5.1, 5.7, 5.10)
ADVERSE REACTIONS
The most common adverse reactions reported are: nausea, vomiting, diarrhea, and abdominal pain (6)
To report SUSPECTED ADVERSE REACTIONS, contact Concordia Pharmaceuticals at 1-877-370-1142 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2022
- Prophylaxis: Begin weekly doses 2 weeks prior to travel to the endemic area, continue weekly doses while in the endemic area, and continue the weekly doses for 4 weeks after leaving the endemic area:
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
1.1 Malaria
1.2 Rheumatoid Arthritis
1.3 Systemic Lupus Erythematosus
1.4 Chronic Discoid Lupus Erythematosus
2 DOSAGE & ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage for Malaria in Adult and Pediatric Patients
2.3 Dosage for Rheumatoid Arthritis in Adults
2.4 Dosage for Systemic Lupus Erythematosus in Adults
2.5 Dosage for Chronic Discoid Lupus Erythematosus in Adults
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy and Ventricular Arrhythmias
5.2 Retinal Toxicity
5.3 Serious Skin Reactions
5.4 Worsening of Psoriasis and Porphyria
5.5 Hematologic Toxicity
5.6 Hemolytic Anemia Associated with G6PD Deficiency
5.7 Skeletal Muscle Myopathy or Neuropathy
5.8 Neuropsychiatric Reactions Including Suicidality
5.9 Hypoglycemia
5.10 Renal Toxicity
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Drugs Prolonging QT Interval and Other Arrhythmogenic Drugs
7.2 Insulin or Other Antidiabetic Drugs
7.3 Drugs that Lower the Seizure Threshold
7.4 Antiepileptics
7.5 Methotrexate
7.6 Cyclosporine
7.7 Digoxin
7.8 Cimetidine
7.9 Rifampicin
7.10 Praziquantel
7.11 Antacids and kaolin
7.12 Ampicillin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Renal or Hepatic Disease
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS & USAGE
1.1 Malaria
PLAQUENIL is indicated in adult and pediatric patients for the:
• Treatment of uncomplicated malaria due to Plasmodium falciparum, Plasmodium malariae, Plasmodium vivax, and Plasmodium ovale.
• Prophylaxis of malaria in geographic areas where chloroquine resistance is not reported.
Limitations of Use:
PLAQUENIL is not recommended for:
• Treatment of complicated malaria.
• Treatment of malaria by chloroquine or hydroxychloroquine-resistant strains of Plasmodium species [see Microbiology (12.4)]
• Treatment of malaria acquired in geographic areas where chloroquine resistance occurs or when the Plasmodium species has not been identified.
• Prophylaxis of malaria in geographic areas where chloroquine resistance occurs.
• Prevention of relapses of P. vivax or P. ovale because it is not active against the hypnozoite liver stage forms of these parasites. For radical cure of P. vivax and P. ovale infections, concomitant therapy with an 8-aminoquinoline drug is necessary [see Microbiology (12.4)] .
For the most current information about drug resistance, refer to the latest recommendations from the Center for Disease Control and Prevention 1 .1.2 Rheumatoid Arthritis
PLAQUENIL is indicated for the treatment of acute and chronic rheumatoid arthritis in adults.
-
2 DOSAGE & ADMINISTRATION
2.1 Important Administration Instructions
Administer PLAQUENIL orally with food or milk. Do not crush or divide the tablets.
2.2 Dosage for Malaria in Adult and Pediatric Patients
PLAQUENIL is not recommended in pediatric patients less than 31 kg because the lowest available strength (200 mg) exceeds the recommended dose for these patients and it cannot be divided.
Prophylaxis
Treatment must start 2 weeks before travel to an endemic area. Advise the patient to take the prophylaxis dosage once a week, staring 2 weeks prior to travel to the endemic area, on the same day every week, continuing the same weekly dose while in the endemic area, and for 4 weeks after leaving the endemic area. The recommended prophylaxis dosage is:
• Adult patients: 400 mg once a week
• Pediatric patients ≥ 31kg: 6.5 mg/kg actual body weight (up to 400 mg) once a week
Treatment of Uncomplicated Malaria
The dosages for the treatment of uncomplicated malaria are:
• Adult patients: Administer 800 mg initially; subsequently administer 400 mg at 6 hours, 24 hours, and 48 hours after the initial dose (total dosage = 2000 mg).
• Pediatric patients ≥ 31 kg: Administer 13 mg/kg (up to 800 mg) initially; subsequently administer 6.5 mg/kg (up to 400 mg) at 6 hours, 24 hours, and 48 hours after the initial dose (total dosage = 31 mg/kg - up to 2000 mg).
For radical cure of P. vivax and P. ovale infections, concomitant therapy with an 8-aminoquinoline drug is necessary [see Microbiology (12.4)] .2.3 Dosage for Rheumatoid Arthritis in Adults
The recommended dosage is:
• Initial dosage: 400 mg to 600 mg daily as a single daily dose or two divided doses. The action of hydroxychloroquine is cumulative and may require weeks to months for maximum therapeutic effect. Daily doses exceeding 5 mg/kg (actual weight) of hydroxychloroquine sulfate increase the incidence of retinopathy [see Warnings and Precautions (5.2)].
• Chronic dosage: 200 mg once daily to 400 mg daily, as a single dose or two divided doses.
Corticosteroids, salicylates, and other antirheumatic agents may be used concomitantly with PLAQUENIL. - 3 DOSAGE FORMS & STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy and Ventricular Arrhythmias
Fatal and life-threatening cases of cardiotoxicity, including cardiomyopathy, have been reported in patients treated with PLAQUENIL. Signs and symptoms of cardiac compromise have occurred during acute and chronic PLAQUENIL treatment. In multiple cases, endomyocardial biopsy showed association of the cardiomyopathy with phospholipidosis in the absence of inflammation, infiltration, or necrosis. Drug-induced phospholipidosis may occur in other organ systems [see Warnings and Precautions( 5.7, 5.10)].
Patients may present with ventricular hypertrophy, pulmonary hypertension and conduction disorders including sick sinus syndrome. ECG findings include atrioventricular, right or left bundle branch block.
PLAQUENIL has a potential to prolong the QT interval. Ventricular arrhythmias (including torsades de pointes) have been reported in PLAQUENIL-treated patients. The magnitude of QT prolongation may increase with increasing concentrations of the drug. Therefore, the recommended dose should not be exceeded [see Adverse Reactions (6), Overdosage (10)]. Avoid PLAQUENIL administration in patients with congenital or documented acquired QT prolongation and/or known risk factors for prolongation of the QT interval such as:
• Cardiac disease, e.g., heart failure, myocardial infarction.
• Proarrhythmic conditions, e.g., bradycardia (< 50 bpm).
• History of ventricular dysrhythmias.
• Uncorrected hypokalemia and/or hypomagnesemia.
• Concomitant administration with QT interval prolonging agents as this may lead to an increased risk for ventricular arrhythmias [see Drug Interactions (7.1)].
Therefore, PLAQUENIL is not recommended in patients taking other drugs that have the potential to prolong the QT interval. Correct electrolyte imbalances prior to use. Monitor cardiac function as clinically indicated during PLAQUENIL therapy. Discontinue PLAQUENIL if cardiotoxicity is suspected or demonstrated by tissue biopsy.5.2 Retinal Toxicity
Irreversible retinal damage was observed in some patients treated with hydroxychloroquine sulfate and it is related to cumulative dosage and treatment duration. In patients of Asian descent, retinal toxicity may first be noticed outside the macula.
Risk factors for retinal damage include daily hydroxychloroquine sulfate dosages ≥5 mg/kg of actual body weight, durations of use greater than five years, renal impairment, use of concomitant drug products such as tamoxifen citrate, and concurrent macular disease.
Within the first year of starting PLAQUENIL, a baseline ocular examination is recommended including best corrected distance visual acuity (BCVA), an automated threshold visual field (VF) of the central 10 degrees (with retesting if an abnormality is noted), and spectral domain ocular coherence tomography (SD-OCT). For patients at higher risk of retinal damage, monitoring should include annual examinations which include BCVA, VF and SD-OCT. For patients without significant risk factors, annual retinal exams can usually be deferred until five years of treatment. In patients of Asian descent, it is recommended that visual field testing be performed in the central 24 degrees instead of the central 10 degrees.
If ocular toxicity is suspected, discontinue PLAQUENIL and monitor the patient closely given that retinal changes and visual disturbances may progress even after cessation of therapy.5.3 Serious Skin Reactions
Serious adverse reactions have been reported with the use of PLAQUENIL including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS syndrome), acute generalized exanthematous pustulosis (AGEP). Monitor for serious skin reactions, especially in patients receiving a drug that may also induce dermatitis. Advise patients to seek medical attention promptly if they experience signs and symptoms of serious skin reactions such as blisters on the skin, eyes, lips or in the mouth, itching or burning, with or without fever [see Warnings and Precautions (5.4), Adverse Reactions (6)] . Discontinue PLAQUENIL if these severe reactions occur.
5.4 Worsening of Psoriasis and Porphyria
Administration of PLAQUENIL in patients with psoriasis may precipitate a severe flare-up of psoriasis. Administration of PLAQUENIL in patients with porphyria may exacerbate porphyria. Avoid PLAQUENIL in patients with psoriasis or porphyria, unless the benefit to the patient outweighs the possible risk.
5.5 Hematologic Toxicity
PLAQUENIL may cause myelosuppression including aplastic anemia, agranulocytosis, leukopenia, or thrombocytopenia. Monitor blood cell counts periodically in patients on prolonged PLAQUENIL therapy. If the patient develops myelosuppression which cannot be attributable to the disease, discontinue the drug.
5.6 Hemolytic Anemia Associated with G6PD Deficiency
Hemolysis has been reported in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. Monitor for hemolytic anemia as this can occur, particularly in association with other drugs that cause hemolysis.
5.7 Skeletal Muscle Myopathy or Neuropathy
Skeletal muscle myopathy or neuropathy leading to progressive weakness and atrophy of proximal muscle groups, depressed tendon reflexes, and abnormal nerve conduction, have been reported. Muscle and nerve biopsies have shown associated phospholipidosis. Drug-induced phospholipidosis may occur in other organ systems [see Warnings and Precautions( 5.1, 5.10) ].
Assess muscle strength and deep tendon reflexes periodically in patients on long-term therapy with PLAQUENIL. Discontinue PLAQUENIL if muscle or nerve toxicity is suspected or demonstrated by tissue biopsy.5.8 Neuropsychiatric Reactions Including Suicidality
Suicidal behavior has been reported in patients treated with PLAQUENIL [see Adverse Reactions (6)]. Alert patients to contact their healthcare provider if they experience new or worsening depression, suicidal thoughts or behavior, or mood changes. The risk and benefit of continued treatment with PLAQUENIL should be assessed for patients who develop these symptoms.
5.9 Hypoglycemia
PLAQUENIL can cause severe and potentially life-threatening hypoglycemia, in the presence or absence of antidiabetic agents [see Drug Interactions (7)]. Measure blood glucose in patients presenting with clinical symptoms suggestive of hypoglycemia and as adjust the antidiabetic treatment as necessary. Warn PLAQUENIL-treated patients about the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia; diabetic patients should monitor their blood sugar levels. Advise patients to seek medical attention if they develop any signs and symptoms of hypoglycemia.
5.10 Renal Toxicity
Proteinuria with or without moderate reduction in glomerular filtration rate have been reported with the use of PLAQUENIL.
Renal biopsy showed phospholipidosis without immune deposits, inflammation, and/or increased cellularity. Physicians should consider phospholipidosis as a possible cause of renal injury in patients with underlying connective tissue disorders who are receiving PLAQUENIL. Drug-induced phospholipidosis may occur in other organ systems [see Warnings and Precautions( 5.1, 5.7) ]. Discontinue PLAQUENIL if renal toxicity is suspected or demonstrated by tissue biopsy. -
6 ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections:
• Cardiomyopathy and Ventricular Arrhythmias [see Warnings and Precautions (5.1)]
• Retinal Toxicity [see Warnings and Precautions (5.2)]
• Serious Skin Reactions [see Warnings and Precautions (5.3)]
• Worsening of Psoriasis and Porphyria [see Warnings and Precautions (5.4)]
• Hematologic Toxicity [see Warnings and Precautions (5.5)]
• Hemolytic Anemia Associated with G6PD [see Warnings and Precautions (5.6)]
• Skeletal Muscle Myopathy or Neuropathy [see Warnings and Precautions (5.7)]
• Neuropsychiatric Reactions Including Suicidality [see Warnings and Precautions (5.8)]
• Hypoglycemia [see Warnings and Precautions (5.9)]
The following adverse reactions have been identified during post-approval use of 4-aminoquinoline drugs, including PLAQUENIL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Blood and lymphatic system disorders: Bone marrow depression, anemia, aplastic anemia, agranulocytosis, leukopenia, thrombocytopenia
- Cardiac disorders: Cardiomyopathy, cardiac failure, QT-interval prolongation, ventricular tachycardia, torsades de pointes, atrioventricular block, bundle branch block, sick sinus syndrome, pulmonary hypertension
- Ear and labyrinth disorders: Vertigo, tinnitus, nystagmus, sensorineural hearing loss
- Eye disorders: Retinopathy, retinal pigmentation changes (typically bull’s eye appearance), visual field defects (paracentral scotomas), macular degeneration, corneal edema, corneal opacities, decreased dark adaptation
- Gastrointestinal disorders: Nausea, vomiting, diarrhea, abdominal pain
- General disorders: Fatigue
- Hepatobiliary disorders: Abnormal liver function tests, fulminant hepatic failure
- Immune system disorders: Urticaria, angioedema, bronchospasm
- Metabolism and nutrition disorders: Anorexia, hypoglycemia, weight loss
- Musculoskeletal and connective tissue disorders: Proximal myopathy, depressed tendon reflexes, abnormal nerve conduction
- Nervous system disorders: Ataxia, dizziness, headache, seizure, extrapyramidal disorders (dystonia, dyskinesia, tremor)
- Psychiatric disorders: Affect/emotional lability, irritability, nervousness, nightmares, psychosis, suicidal ideation, suicidal behavior
- Skin and subcutaneous tissue disorders: Alopecia, hair color changes, rash, pruritus, photosensitivity, psoriasis exacerbation, hyperpigmentation, exfoliative dermatitis, erythema multiforme, acute generalized exanthematous pustulosis, Drug Rash with Eosinophilia and Systemic Symptoms (DRESS syndrome), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) -
7 DRUG INTERACTIONS
7.1 Drugs Prolonging QT Interval and Other Arrhythmogenic Drugs
PLAQUENIL prolongs the QT interval. There may be an increased risk of inducing ventricular arrhythmias if PLAQUENIL is used concomitantly with other arrhythmogenic drugs. Therefore, PLAQUENIL is not recommended in patients taking other drugs that have the potential to prolong the QT interval or are arrhythmogenic [see Warnings and Precautions (5.1)].
7.2 Insulin or Other Antidiabetic Drugs
PLAQUENIL may enhance the effects of insulin and antidiabetic drugs, and consequently increase the hypoglycemic risk. Therefore, a decrease in dosage of insulin and other antidiabetic drugs may be necessary [see Warnings and Precautions (5.8)].
7.3 Drugs that Lower the Seizure Threshold
PLAQUENIL can lower the seizure threshold. Co-administration of PLAQUENIL with other antimalarials known to lower the seizure threshold (e.g., mefloquine) may increase the risk of seizures.
7.4 Antiepileptics
The activity of antiepileptic drugs might be impaired if co-administered with PLAQUENIL.
7.5 Methotrexate
Concomitant use of PLAQUENIL and methotrexate may increase the incidence of adverse reactions.
7.6 Cyclosporine
An increased plasma cyclosporin level was reported when cyclosporin and PLAQUENIL were co-administered. Monitor serum cyclosporine levels closely in patients receiving combined therapy.
7.7 Digoxin
Concomitant PLAQUENIL and digoxin therapy may result in increased serum digoxin levels. Monitor serum digoxin levels closely in patients receiving combined therapy.
7.8 Cimetidine
Concomitant use of cimetidine resulted in a 2-fold increase of exposure of chloroquine, which is structurally related to hydroxychloroquine. Interaction of cimetidine with hydroxychloroquine cannot be ruled out. Avoid concomitant use of cimetidine
7.9 Rifampicin
Lack of efficacy of hydroxychloroquine was reported when rifampicin was concomitantly administered. Avoid concomitant use of rifampicin
7.10 Praziquantel
Chloroquine has been reported to reduce the bioavailability of praziquantel. Interaction of praziquantel with hydroxychloroquine cannot be ruled out.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to PLAQUENIL during pregnancy. Encourage patients to register by contacting 1-877-311-8972.
Risk Summary
Prolonged clinical experience over decades of use and available data from published epidemiologic and clinical studies with PLAQUENIL use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal, or fetal outcomes (see Data). There are risks to the mother and fetus associated with untreated or increased disease activity from malaria, rheumatoid arthritis, and systemic lupus erythematosus in pregnancy (see Clinical Considerations). Animal reproduction studies were not conducted with hydroxychloroquine.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo-Fetal Risk
Malaria: Malaria during pregnancy increases the risk for adverse pregnancy outcomes, including maternal anemia, prematurity, spontaneous abortion, and stillbirth.
Rheumatoid Arthritis: Published data suggest that increased disease activity is associated with the risk of developing adverse pregnancy outcomes in women with rheumatoid arthritis Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2500 g) infants, and small for gestational age at birth.
Systemic Lupus Erythematosus: Pregnant women with systemic lupus erythematosus, especially those with increased disease activity, are at increased risk of adverse pregnancy outcomes, including spontaneous abortion, fetal death, preeclampsia, preterm birth, and intrauterine growth restriction. Passage of maternal auto-antibodies across the placenta may result in neonatal illness, including neonatal lupus and congenital heart block.
Data
Human Data
Data from published epidemiologic and clinical studies have not established an association with PLAQUENIL use during pregnancy and major birth defects, miscarriage, or adverse maternal or fetal outcomes. Hydroxychloroquine readily crosses the placenta with cord blood levels corresponding to maternal plasma levels. No retinal toxicity, ototoxicity, cardiotoxicity, or growth and developmental abnormalities have been observed in children who were exposed to hydroxychloroquine in utero. Available epidemiologic and clinical studies have methodological limitations including small sample size and study design.8.2 Lactation
Risk Summary
Published lactation data report that hydroxychloroquine is present in human milk at low levels. No adverse reactions have been reported in breastfed infants. No retinal toxicity, ototoxicity, cardiotoxicity, or growth and developmental abnormalities have been observed in children who were exposed to hydroxychloroquine through breastmilk. There is no information on the effect of hydroxychloroquine on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for PLAQUENIL and any potential adverse effects on the breastfed child from PLAQUENIL or from the underlying maternal condition.8.4 Pediatric Use
The safety and effectiveness of PLAQUENIL have been established in pediatric patients for the treatment of uncomplicated malaria due to P. falciparum, P. malariae, P. vivax, and P. ovale, as well as for the prophylaxis of malaria in geographic areas where chloroquine resistance is not reported. However, this product cannot be directly administered to pediatric patients weighing less than 31 kg because the film-coated tablets cannot be crushed or divided [see Dosage and Administration (2.1, 2.2)] .
The safety and effectiveness of PLAQUENIL have not been established in pediatric patients for the treatment of rheumatoid arthritis, chronic discoid lupus erythematosus, or systemic lupus erythematosus.8.5 Geriatric Use
Clinical trials of PLAQUENIL did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients. Nevertheless, this drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. In general, dose selection in geriatric patients should start with the lowest recommended dose, taking into consideration the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
PLAQUENIL overdosage symptoms have an onset within 1–3 hours of ingestion. The following have been reported with PLAQUENIL overdosage:
• Cardiovascular toxicity, including QRS or QTc prolongation, ventricular tachycardia, ventricular fibrillation, torsade de pointes, atrioventricular block, cardiac arrest and death.
• Life-threatening hypotension is common.
• Severe hypokalemia secondary to an intracellular shift is common in severe toxicity.
• Central nervous system (CNS) depression, seizures, visual disturbances, transient blindness, and coma may occur.
Gastrointestinal decontamination procedures warrant consideration in patients that present within the first hour post-ingestion. If the level of consciousness rapidly deteriorates in severe poisoning, consider intubation before gastrointestinal decontamination procedures. Monitor plasma potassium levels and manage accordingly. Hemofiltration, hemodialysis, and hemoperfusion are not of benefit.
Consider contacting a poison center (1-800-221-2222) or a medical toxicologist for overdosage management recommendations. -
11 DESCRIPTION
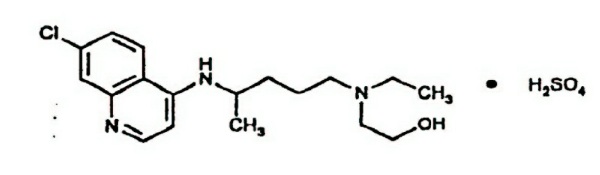
PLAQUENIL (hydroxychloroquine sulfate) is an antimalarial and antirheumatic drug, chemically described as 2-[[4-[(7-Chloro-4- quinolyl) amino]pentyl] ethylamino]ethanol sulfate (1:1) with the molecular formula C 18H 26ClN 3O•H 2SO 4. The molecular weight of hydroxychloroquine sulfate is 433.95. Its structural formula is:
Hydroxychloroquine sulfate is a white or off-white crystalline powder, freely soluble in water; practically soluble in alcohol, chloroform, and ether.
PLAQUENIL (hydroxychloroquine sulfate) tablets for oral administration contain 200 mg hydroxychloroquine sulfate (equivalent to 155 mg of hydroxychloroquine) and the following inactive ingredients: black iron oxide, carnauba wax, corn starch, dibasic calcium phosphate, hydroxypropyl methylcellulose, magnesium stearate, polyethylene glycol 400, polysorbate 80, titanium dioxide. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Malaria
Hydroxychloroquine is a 4-aminoquinoline antimalarial [see Microbiology (12.4)] and antirheumatic agent.
Rheumatoid Arthritis, Systemic Lupus Erythematosus and Chronic Discoid Lupus Erythematosus
The mechanisms underlying the anti-inflammatory and immunomodulatory effects of PLAQUENIL in the treatment of rheumatoid arthritis, chronic discoid lupus erythematosus and systemic lupus erythematosus are not fully known.12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of hydroxychloroquine have not been fully characterized.
12.3 Pharmacokinetics
Absorption
Following a single 200 mg oral dose of PLAQUENIL to healthy male volunteers, whole blood hydroxychloroquine Cmax was 129.6 ng/mL (plasma Cmax was 50.3 ng/mL) with Tmax of 3.3 hours (plasma Tmax 3.7 hours). Following a single oral PLAQUENIL dose of 200 mg, the mean fraction of the dose absorbed was 0.74 (compared to administration of 155 mg of hydroxychloroquine intravenous infusion). Peak blood concentrations of metabolites were observed at the same time as peak levels of hydroxychloroquine.
After administration of single 155 mg and 310 mg intravenous doses, peak blood concentrations ranged from 1161 ng/mL to 2436 ng/mL (mean 1918 ng/mL) following the 155 mg infusion and 6 months following the 310 mg infusion. Pharmacokinetic parameters were not significantly different over the therapeutic dose range of 155 mg and 310 mg indicating linear kinetics.
In patients with rheumatoid arthritis, there was large variability as to the fraction of the dose absorbed (i.e. 30 to 100%), and mean hydroxychloroquine levels were significantly higher in patients with less disease activity.
Distribution
Plaquenil is extensively distributed to tissues.
Elimination
A half-life of 123.5 days in plasma were observed following a single 200 mg oral PLAQUENIL dose to healthy male volunteers. Urine hydroxychloroquine levels were still detectable after 3 months with approximately 10% of the dose excreted as the parent drug. Results following a single dose of a 200 mg tablet versus i.v. infusion (155 mg), demonstrated a half-life of about 40 days and a large volume of distribution. Following chronic oral administration of hydroxychloroquine, the absorption half-life was approximately 3 to 4 hours and the terminal half-life ranged from 40 to 50 days.
Metabolism
Significant levels of three metabolites, desethylhydroxychloroquine (DHCQ), desethylchloroquine (DCQ), and bidesethylhydroxychloroquine (BDCQ) were found in plasma and blood, with DHCQ being the major metabolite.
Excretion
Renal clearance in patients with rheumatoid arthritis treated with PLAQUENIL for at least 6 months was similar to that in single dose studies in healthy volunteers, suggesting that no change in clearance occurred with chronic dosing. Renal clearance of unchanged drug was approximately 16% to 30%.12.4 Microbiology
Mechanism of Action in Malaria
The precise mechanism by which hydroxychloroquine exhibits activity against Plasmodium is not known. Hydroxychloroquine is a weak base and may exert its effect by concentrating in the acid vesicles of the parasite and inhibiting polymerization of heme. It can also inhibit certain enzymes by its interaction with DNA.
Antimicrobial ActivityHydroxychloroquine is active against the erythrocytic forms of chloroquine sensitive strains of P. falciparum, P. malariae, P. vivax, and P. ovale. Hydroxychloroquine is not active against the gametocytes and exoerythrocytic forms including the hypnozoite liver stage forms of P. vivax and P. ovale.
Drug ResistanceP. falciparum strains exhibiting reduced susceptibility to chloroquine also show reduced susceptibility to hydroxychloroquine. Resistance of Plasmodium parasites to chloroquine is widespread [see Indications and Usage (1.1)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with hydroxychloroquine. No animal studies have been performed to evaluate the potential effects of hydroxychloroquine on reproduction or development, or to determine potential effects on fertility in males or females.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
PLAQUENIL tablets contain 200 mg hydroxychloroquine sulfate (equivalent to 155 mg base). White to off-white film coated, no score, tablet imprinted with “PLAQUENIL” on one face in black ink. The tablets are available in bottles of:
• 60 tablets - NDC 59212-562-60
• 100 tablets - NDC 59212-562-10 and NDC 59212-562-11 -
17 PATIENT COUNSELING INFORMATION
Important Administration Instructions
Advise the patient to take PLAQUENIL with food or milk and not to crush or divide the tablet.
Cardiomyopathy and Ventricular Arrhythmias
Inform the patient that serious cardiac effects, life-threatening and fatal cases have been reported with use of PLAQUENIL Advise patients to seek medical attention immediately if they experience any symptoms of heart rhythm changes including fast or irregular heartbeat, lightheadedness, dizziness, or syncope [see Warnings and Precautions (5.1)] .
Retinal Toxicity
Inform the patient that irreversible retinal damage has been observed in some patients with the use of PLAQUENIL. Advise patients of the importance of the ophthalmology visits for monitoring their eyes. Instruct patients to seek medical attention promptly if they experience decreased vision or decreased dark adaptation [see Warnings and Precautions (5.2)] .
Serious Skin Reactions
Inform the patient that severe, life-threatening skin reactions have been reported with the use of PLAQUENIL. Advise the patient to seek medical attention immediately if experiencing any of the following signs and symptoms: blisters on the skin, eyes, lips or in the mouth, itching or burning, with or without fever [see Warnings and Precautions (5.3)] .
Skeletal Muscle Myopathy or Neuropathy
Inform the patient that muscle weakness and atrophy has been reported with PLAQUENIL use Advise patients to report to the physician symptoms of muscle weakness [see Warnings and Precautions (5.7)] .
Neuropsychiatric Reactions Including Suicidality
Alert patients to seek medical attention immediately if they experience new or worsening depression, suicidal thoughts, or other mood changes [see Warnings and Precautions (5.8)] .
Hypoglycemia
Inform the patient that PLAQUENIL has been associated with severe hypoglycemia. Advise the patient to monitor blood sugar levels if possible and to seek medical attention if experiencing any of the signs and symptoms of hypoglycemia such as sweating, shakiness, weakness, dizziness, tachycardia, nausea, blurred vision, confusion, fainting, or loss of consciousness [see Warnings and Precautions (5.9)] .
Pregnancy
Inform the patient that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to PLAQUENIL during pregnancy. Encourage patients to register by contacting 1-877-311-8972 [see Use in Specific Populations (8.1)] .
Mfd. for:
Concordia Pharmaceuticals
Distributed by:
Amdipharm Limited
17 Northwood House
Dublin 9, Ireland
©2015 All rights reserved. -
Repackaging Information
Please reference the HOW SUPPLIED section listed above for a description of individual drug products listed below. This drug product has been received by Aphena Pharma Solutions - Tennessee, LLC in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
200mg
NDC 71610-473-30, Bottles of 30 Tablets
NDC 71610-473-53, Bottles of 60 Tablets
NDC 71610-473-80, Bottles of 180 Tablets
NDC 71610-473-74, Bottles of 500 Tablets
NDC 71610-473-04, Bottles of 4000 TabletsStore between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20240404AMH - PRINCIPAL DISPLAY PANEL - 200 mg
-
INGREDIENTS AND APPEARANCE
PLAQUENIL
hydroxychloroquine sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71610-473(NDC:59212-562) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROXYCHLOROQUINE SULFATE (UNII: 8Q2869CNVH) (HYDROXYCHLOROQUINE - UNII:4QWG6N8QKH) HYDROXYCHLOROQUINE SULFATE 200 mg Inactive Ingredients Ingredient Name Strength CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CARNAUBA WAX (UNII: R12CBM0EIZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color white Score no score Shape DOUBLE CIRCLE (peanut shaped) Size 12mm Flavor Imprint Code PLAQUENIL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71610-473-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2020 2 NDC:71610-473-53 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2020 3 NDC:71610-473-80 180 in 1 BOTTLE; Type 0: Not a Combination Product 12/08/2020 4 NDC:71610-473-74 500 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2023 5 NDC:71610-473-04 4000 in 1 BOTTLE; Type 0: Not a Combination Product 04/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA009768 06/28/2013 Labeler - Aphena Pharma Solutions - Tennessee, LLC (128385585) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions - Tennessee, LLC 128385585 repack(71610-473)


