Label: BACITRACIN ZINC ointment
- NDC Code(s): 0404-0172-50
- Packager: Henry Schein
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use(s)
-
Warnings
For External Use Only
Do not use
• In the eyes or over large areas of the body
• If you are allergic to any of the ingredients
• Longer than 1 week unless directed by a doctor
Ask a doctor or pharmacist before use if you have
deep or puncture wounds, animal bites, or serious burns
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- Label
-
INGREDIENTS AND APPEARANCE
BACITRACIN ZINC
bacitracin zinc ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0404-0172 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 500 [iU] in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PARAFFIN (UNII: I9O0E3H2ZE) WHITE PETROLATUM (UNII: B6E5W8RQJ4) Product Characteristics Color Score Shape FREEFORM Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0404-0172-50 1728 in 1 CASE 11/07/2022 1 144 in 1 BOX 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 11/07/2022 Labeler - Henry Schein (012430880)

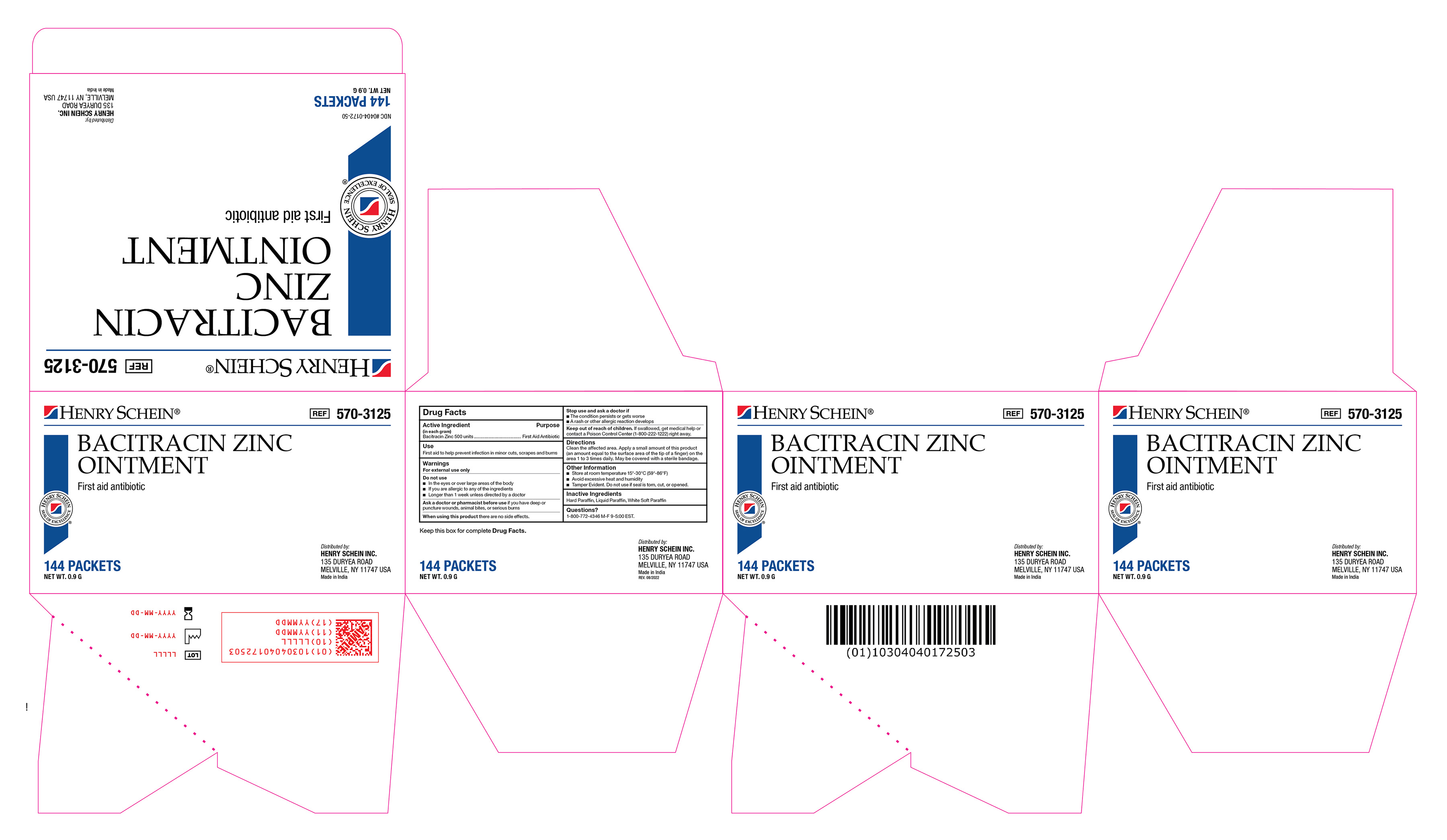
 Bacitracin Zinc
Bacitracin Zinc