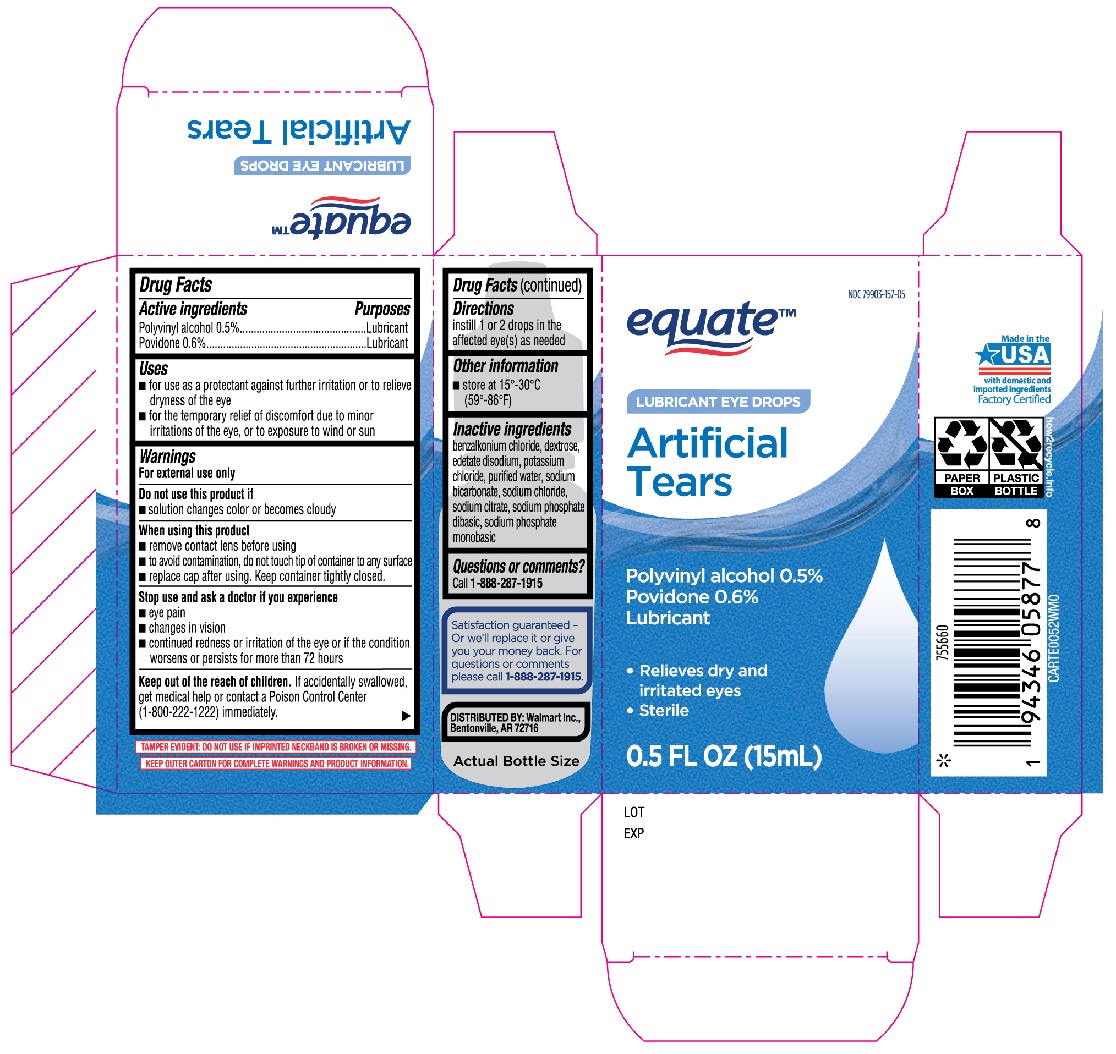
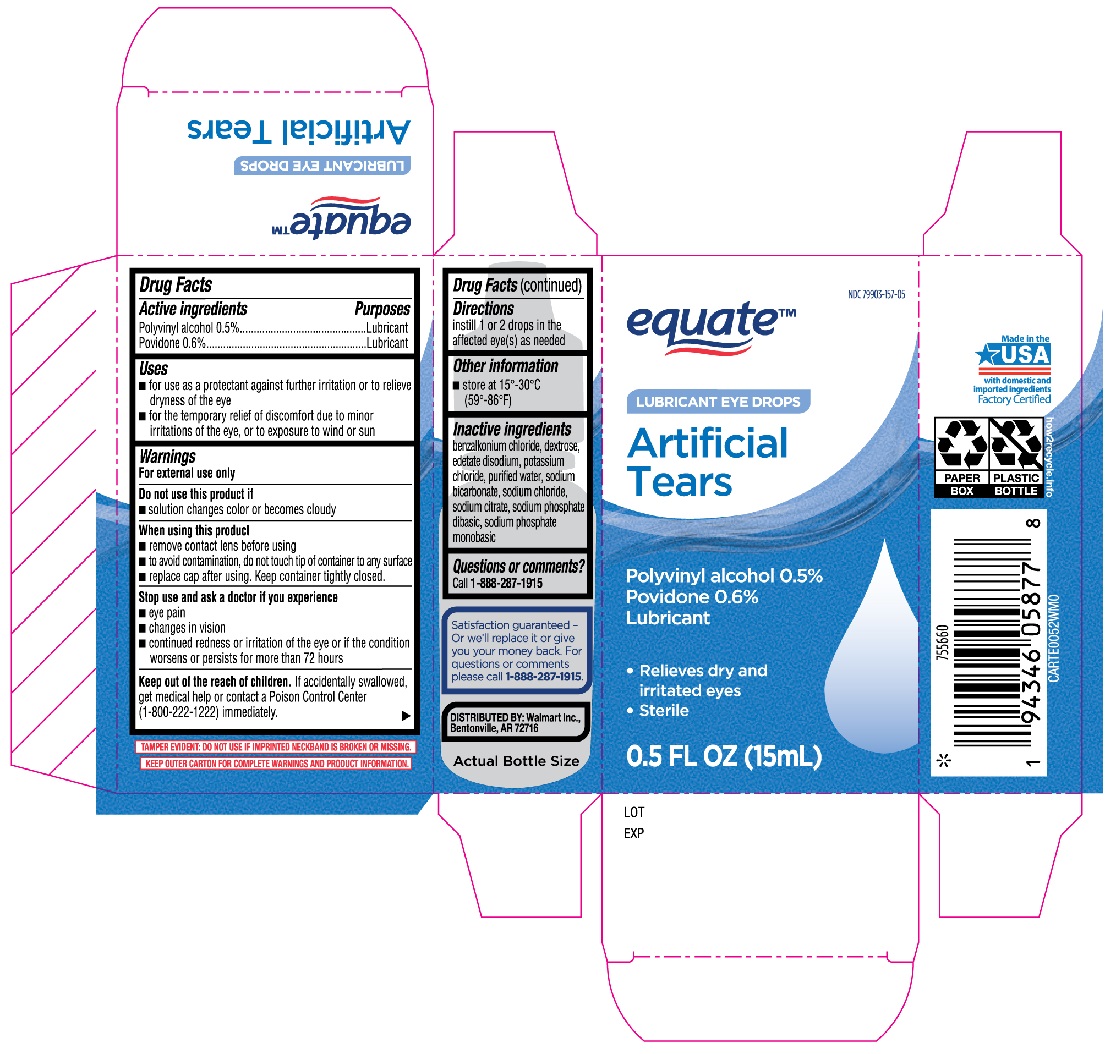
Label: EQUATE ARTIFICIAL TEARS- polyvinyl alcohol, povidone solution/ drops
- NDC Code(s): 79903-157-05
- Packager: Walmart, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- remove contact lens before using
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using. Keep container tightly closed.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Equate Artificial Tears 15mL
-
INGREDIENTS AND APPEARANCE
EQUATE ARTIFICIAL TEARS
polyvinyl alcohol, povidone solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-157 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE (UNII: FZ989GH94E) (POVIDONE, UNSPECIFIED - UNII:FZ989GH94E) POVIDONE 0.6 g in 100 mL POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) (POLYVINYL ALCOHOL, UNSPECIFIED - UNII:532B59J990) POLYVINYL ALCOHOL, UNSPECIFIED 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) DEXTROSE (UNII: IY9XDZ35W2) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) POTASSIUM CHLORIDE (UNII: 660YQ98I10) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-157-05 1 in 1 BOX 02/16/2023 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 02/16/2023 Labeler - Walmart, Inc. (051957769) Registrant - KC Pharmaceuticals, Inc. (174450460) Establishment Name Address ID/FEI Business Operations KC Pharmaceuticals, Inc. 174450460 manufacture(79903-157) , pack(79903-157) , label(79903-157)