Label: PURELL CS FOAM HANDWASH 2% CHG- chlorhexidine gluconate 2% solution liquid
- NDC Code(s): 21749-226-40
- Packager: GOJO Industries, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated October 26, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Uses
- Active Ingredient
- Purpose
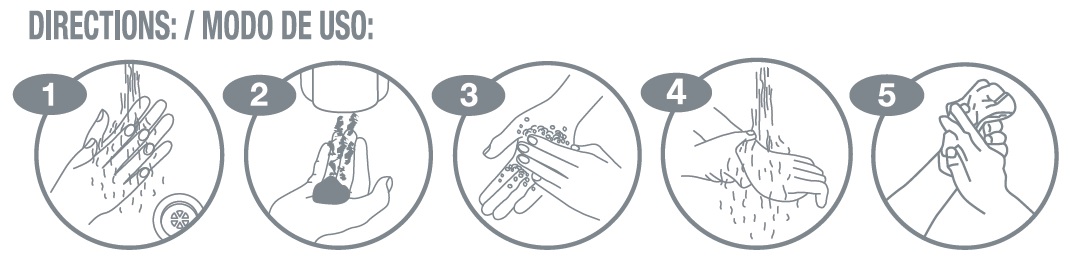
- Directions
- Warnings
- Do not use
-
When using this product
keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if placed or kept in the eye during surgical procedures or may cause deafness when instilled in the middle of the ear through perforated eardrums. If solution should contact these areas, rinse out promptly and thoroughly with water.
- Stop use and ask a doctor if
- Keep out of reach of children
- Allergy Alert
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PURELL CS FOAM HANDWASH 2% CHG
chlorhexidine gluconate 2% solution liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21749-226 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) ISOPROPYL ALCOHOL (UNII: ND2M416302) COCO DIETHANOLAMIDE (UNII: 92005F972D) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21749-226-40 1200 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/13/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 10/13/2022 Labeler - GOJO Industries, Inc. (004162038) Registrant - Xttrium Laboratories, Inc. (007470579) Establishment Name Address ID/FEI Business Operations Xttrium Laboratories, Inc. 007470579 manufacture(21749-226)