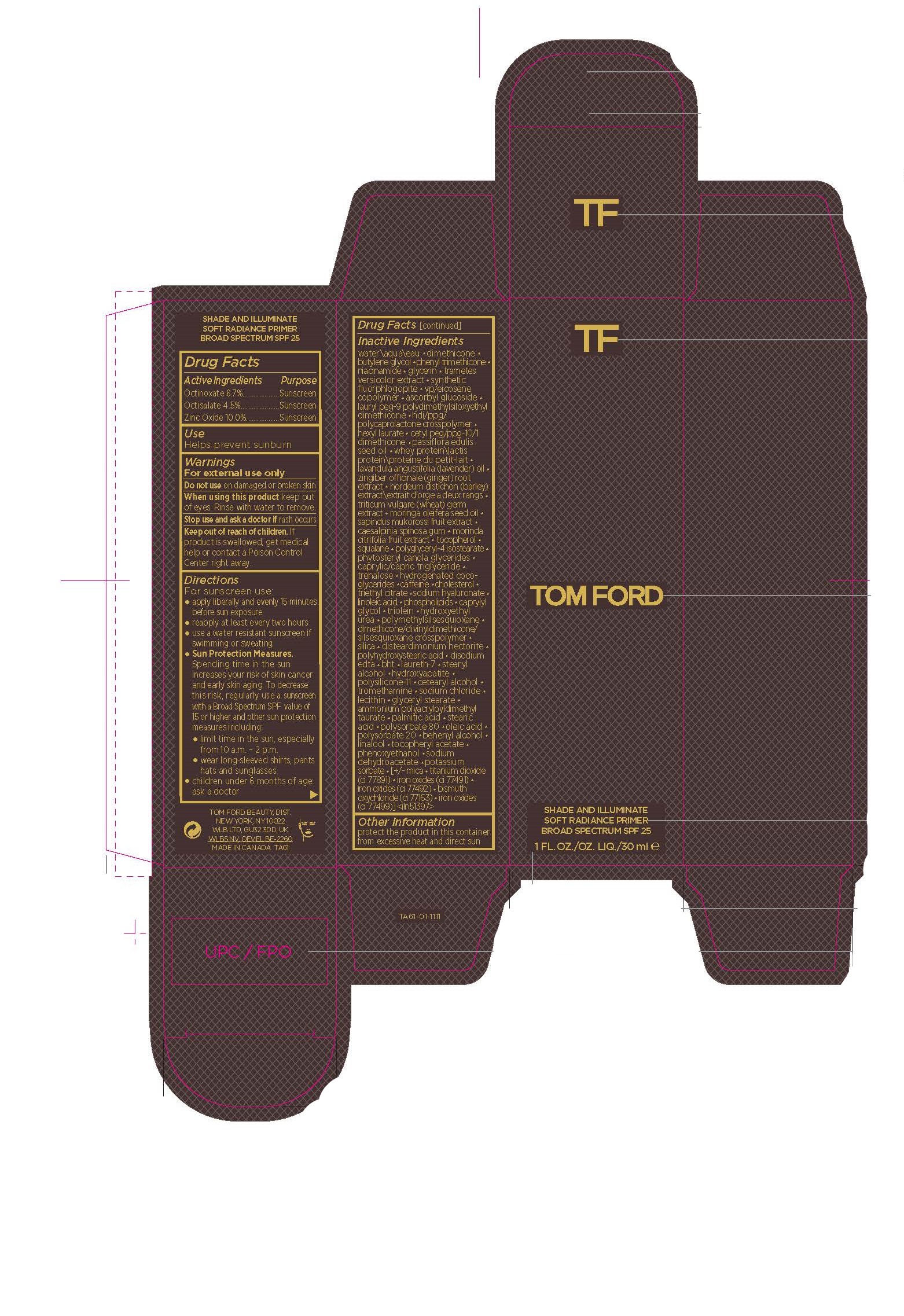
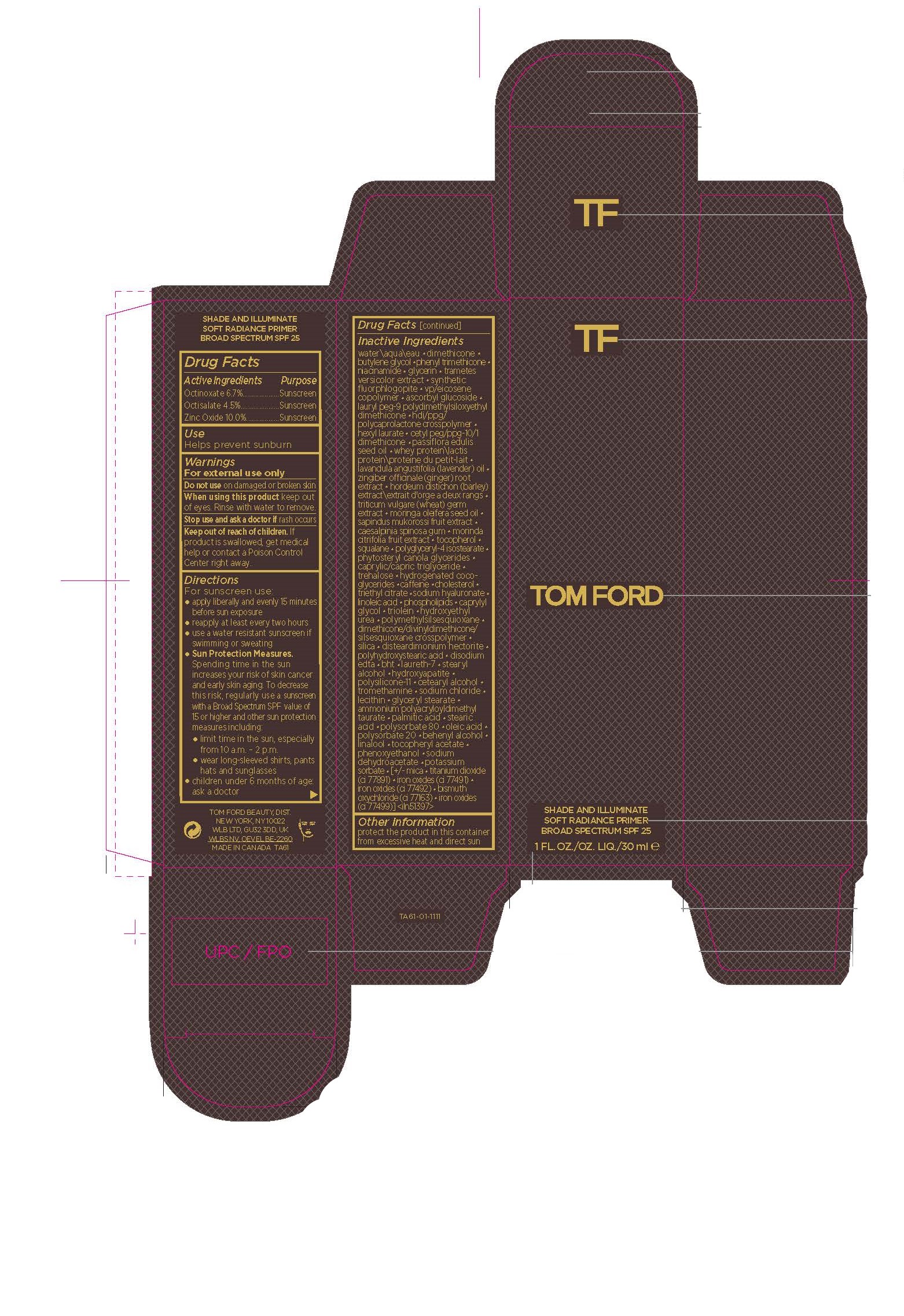
Label: SHADE AND ILLUMINATE SOFT RAD PRMR SPF- octinoxate,octisalate,zinc oxide cream
- NDC Code(s): 76398-013-01, 76398-013-02
- Packager: TOM FORD BEAUTY DIST
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- USE
- Warnings
-
Directions
For sunscreen use:
• apply liberally and evenly 15 minutes
before sun exposure
• reapply at least every two hours
• use a water resistant sunscreen if
swimming or sweating
• Sun Protection Measures.
Spending time in the sun
increases your risk of skin cancer
and early skin aging. To decrease
this risk, regularly use a sunscreen
with a Broad Spectrum SPF value of
15 or higher and other sun protection
measures including:
• limit time in the sun, especially
from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants
hats and sunglasses
• children under 6 months of age:
ask a doctor -
Inactive Ingredients
WATER\AQUA\EAU [] DIMETHICONE [] BUTYLENE GLYCOL [] PHENYL TRIMETHICONE [] NIACINAMIDE [] GLYCERIN [] TRAMETES VERSICOLOR EXTRACT [] SYNTHETIC FLUORPHLOGOPITE [] VP/EICOSENE COPOLYMER [] ASCORBYL GLUCOSIDE [] LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE [] HDI/PPG/POLYCAPROLACTONE CROSSPOLYMER [] HEXYL LAURATE [] CETYL PEG/PPG-10/1 DIMETHICONE [] PASSIFLORA EDULIS SEED OIL [] WHEY PROTEIN\LACTIS PROTEIN\PROTEINE DU PETIT-LAIT [] LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL [] ZINGIBER OFFICINALE (GINGER) ROOT EXTRACT [] HORDEUM DISTICHON (BARLEY) EXTRACT\EXTRAIT D'ORGE A DEUX RANGS [] TRITICUM VULGARE (WHEAT) GERM EXTRACT [] MORINGA OLEIFERA SEED OIL [] SAPINDUS MUKOROSSI FRUIT EXTRACT [] CAESALPINIA SPINOSA GUM [] MORINDA CITRIFOLIA FRUIT EXTRACT [] TOCOPHEROL [] SQUALANE [] POLYGLYCERYL-4 ISOSTEARATE [] PHYTOSTERYL CANOLA GLYCERIDES [] CAPRYLIC/CAPRIC TRIGLYCERIDE [] TREHALOSE [] HYDROGENATED COCO-GLYCERIDES [] CAFFEINE [] CHOLESTEROL [] TRIETHYL CITRATE [] SODIUM HYALURONATE [] LINOLEIC ACID [] PHOSPHOLIPIDS [] CAPRYLYL GLYCOL [] TRIOLEIN [] HYDROXYETHYL UREA [] POLYMETHYLSILSESQUIOXANE [] DIMETHICONE/DIVINYLDIMETHICONE/SILSESQUIOXANE CROSSPOLYMER [] SILICA [] DISTEARDIMONIUM HECTORITE [] POLYHYDROXYSTEARIC ACID [] DISODIUM EDTA [] BHT [] LAURETH-7 [] STEARYL ALCOHOL [] HYDROXYAPATITE [] POLYSILICONE-11 [] CETEARYL ALCOHOL [] TROMETHAMINE [] SODIUM CHLORIDE [] LECITHIN [] GLYCERYL STEARATE [] AMMONIUM POLYACRYLOYLDIMETHYL TAURATE [] PALMITIC ACID [] STEARIC ACID [] POLYSORBATE 80 [] OLEIC ACID [] POLYSORBATE 20 [] BEHENYL ALCOHOL [] LINALOOL [] TOCOPHERYL ACETATE [] PHENOXYETHANOL [] SODIUM DEHYDROACETATE [] POTASSIUM SORBATE [] [+/- MICA [] TITANIUM DIOXIDE (CI 77891) [] IRON OXIDES (CI 77491) [] IRON OXIDES (CI 77492) [] BISMUTH OXYCHLORIDE (CI 77163) [] IRON OXIDES (CI 77499)] <ILN51397>
- Other Information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SHADE AND ILLUMINATE SOFT RAD PRMR SPF
octinoxate,octisalate,zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76398-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 100 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 67 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength TREHALOSE (UNII: B8WCK70T7I) CAFFEINE (UNII: 3G6A5W338E) CHOLESTEROL (UNII: 97C5T2UQ7J) MORINGA OLEIFERA SEED OIL (UNII: REM6A5QMC0) SAPINDUS MUKOROSSI FRUIT (UNII: 66H9NW427Y) CAESALPINIA SPINOSA RESIN (UNII: WL3883U2PO) MORINDA CITRIFOLIA FRUIT (UNII: 7829X3G2X5) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) TRAMETES VERSICOLOR FRUITING BODY (UNII: 4C900477MT) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) OLEIC ACID (UNII: 2UMI9U37CP) POLYSORBATE 20 (UNII: 7T1F30V5YH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MICA (UNII: V8A1AW0880) FERROSOFERRIC OXIDE (UNII: XM0M87F357) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) LAVENDER OIL (UNII: ZBP1YXW0H8) LINOLEIC ACID (UNII: 9KJL21T0QJ) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HEXYL LAURATE (UNII: 4CG9F9W01Q) GINGER (UNII: C5529G5JPQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HYDROGENATED COCO-GLYCERIDES (UNII: XDD37N2GPR) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SQUALANE (UNII: GW89575KF9) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) PASSIFLORA EDULIS SEED OIL (UNII: F3VOA31UHQ) WHEY (UNII: 8617Z5FMF6) BARLEY (UNII: 5PWM7YLI7R) WHEAT GERM (UNII: YR3G369F5A) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCERYL TRIOLEATE (UNII: O05EC62663) HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) DIMETHICONE/DIVINYLDIMETHICONE/SILSESQUIOXANE CROSSPOLYMER (UNII: T3064TZ75A) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LAURETH-7 (UNII: Z95S6G8201) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TROMETHAMINE (UNII: 023C2WHX2V) SODIUM CHLORIDE (UNII: 451W47IQ8X) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) AMMONIUM POLYACRYLOYLDIMETHYL TAURATE (55000 MPA.S) (UNII: F01RIY4371) PALMITIC ACID (UNII: 2V16EO95H1) STEARIC ACID (UNII: 4ELV7Z65AP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) DOCOSANOL (UNII: 9G1OE216XY) LINALOOL, (+/-)- (UNII: D81QY6I88E) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) NIACINAMIDE (UNII: 25X51I8RD4) GLYCERIN (UNII: PDC6A3C0OX) EICOSYL POVIDONE (UNII: XQQ9MKE2BJ) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76398-013-01 1 in 1 CARTON 10/14/2022 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:76398-013-02 1 in 1 CARTON 10/14/2022 11/30/2023 2 3.6 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/14/2022 Labeler - TOM FORD BEAUTY DIST (005914387) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 202952982 manufacture(76398-013) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 204132062 pack(76398-013) , label(76398-013)

 TOM FORD
TOM FORD