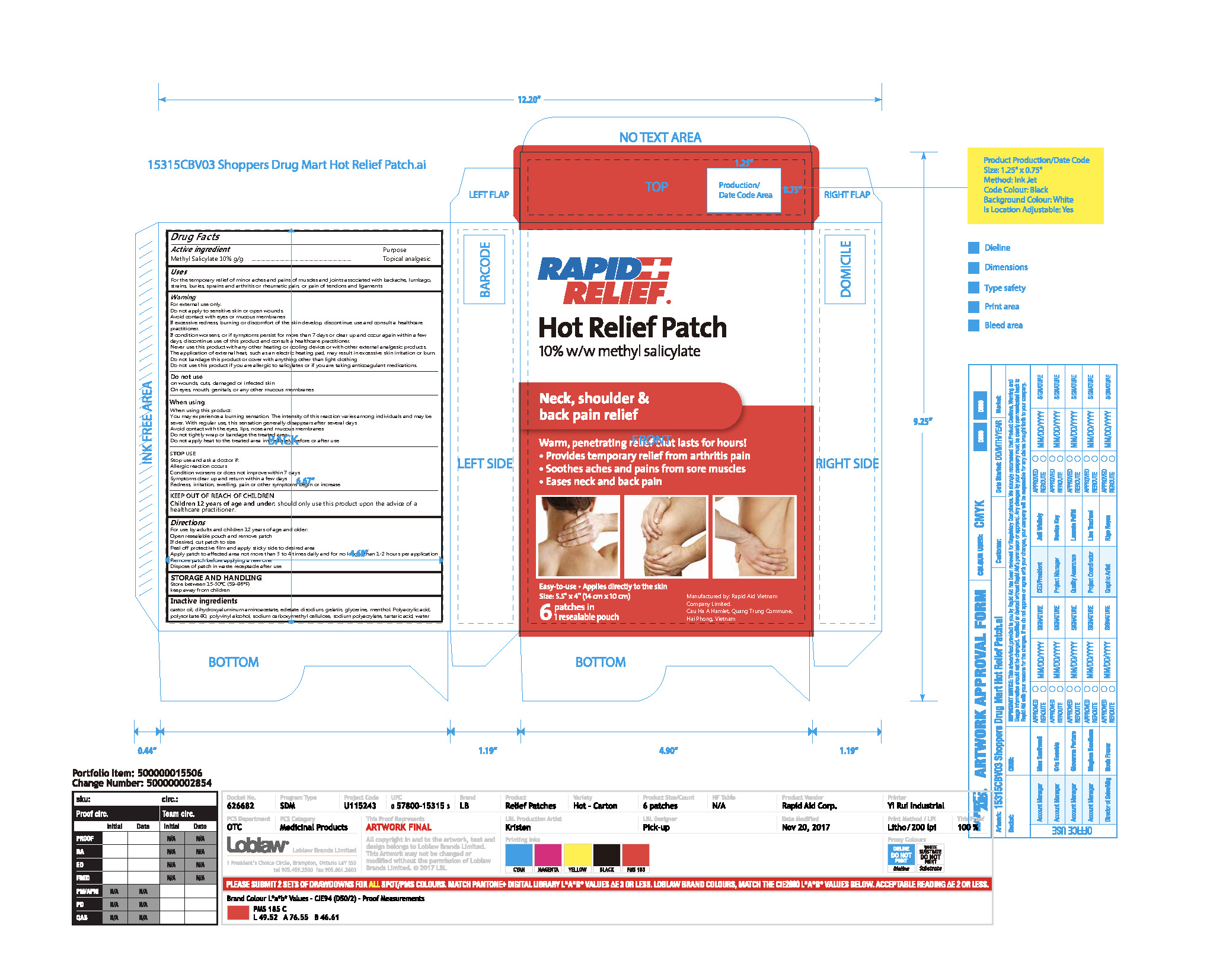
Label: RAPID RELIEF HOT RELIEF PATCH- methyl salicylate patch
- NDC Code(s): 83569-003-01
- Packager: RAPID AID VIET NAM CO., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
-
Warnings
For external use only.
Do not apply to sensitive skin or open wounds.
Avoid contact with eyes or mucous membranes
If excessive redness, burning or discomfort of the skin develop, discontinue use and consult a healthcare practitioner.
If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a healthcare practitioner.
Never use this product with any other heating or cooling device or with other external analgesic products.
The application of external heat, such as an electric heating pad, may result in excessive skin irritation or burn.
Do not bandage this product or cover with anything other than light clothing
Do not use this product if you are allergic to salicylates or if you are taking anticoagulant medications. - Do not use
-
When using this product:
You may experience a burning sensation. The intensity of this reaction varies among individuals and may be sever. With regular use, this sensation generally disappears after several days.
Avoid contact with the eyes, lips, nose and mucous membranes
Do not tightly wrap or bandage the treated area
Do not apply heat to the treated area immediately before or after use - Stop use and ask a doctor if:
- KEEP OUT OF REACH OF CHILDREN
-
Directions
For use by adults and children 12 years of age and older:
Open resealable pouch and remove patch
If desired, cut patch to size
Peel off protective film and apply sticky side to desired area
Apply patch to affected area not more than 3 to 4 times daily and for no longer than 1-2 hours per application
Remove patch before applying a new one
Dispose of patch in waste receptacle after use - STORAGE AND HANDLING
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
RAPID RELIEF HOT RELIEF PATCH
methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83569-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methyl Salicylate (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) Methyl Salicylate 10 g in 100 g Inactive Ingredients Ingredient Name Strength menthol (UNII: L7T10EIP3A) castor oil (UNII: D5340Y2I9G) gelatin (UNII: 2G86QN327L) tartaric acid (UNII: W4888I119H) Polysorbate 80 (UNII: 6OZP39ZG8H) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYACRYLIC ACID (450000 MW) (UNII: KD3S7H73D3) glycerin (UNII: PDC6A3C0OX) water (UNII: 059QF0KO0R) edetate disodium (UNII: 7FLD91C86K) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) dihydroxyaluminum aminoacetate (UNII: DO250MG0W6) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83569-003-01 1 in 1 BOX 03/06/2024 1 6 in 1 POUCH 1 10 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/06/2024 Labeler - RAPID AID VIET NAM CO., LTD (673067008) Registrant - RAPID AID VIET NAM CO., LTD (673067008) Establishment Name Address ID/FEI Business Operations RAPID AID VIET NAM CO., LTD 673067008 manufacture(83569-003)